Here are the essential concepts you must grasp in order to answer the question correctly.
Alkane Structure
Alkanes are saturated hydrocarbons consisting only of carbon and hydrogen atoms, connected by single bonds. Their general formula is CnH2n+2, where 'n' is the number of carbon atoms. Understanding the structural representation of alkanes, including branched and straight-chain forms, is essential for identifying and drawing the correct structures based on given names.
Recommended video:
IUPAC Nomenclature
The International Union of Pure and Applied Chemistry (IUPAC) nomenclature provides a systematic way to name organic compounds. For alkanes, names reflect the number of carbon atoms and the structure, including prefixes for branches (like 'iso-' for isopropyl) and suffixes indicating the type of compound. Familiarity with these naming conventions is crucial for accurately interpreting and constructing molecular structures.
Recommended video:
The different parts of an IUPAC name
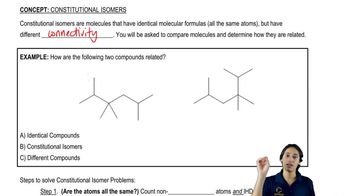
Structural Isomers
Structural isomers are compounds that share the same molecular formula but differ in the arrangement of atoms. In the case of alkanes, this can lead to various structural forms, such as branched and unbranched chains. Recognizing the potential for isomerism is vital for drawing multiple structures that fit a given description, as seen in the examples of isopropylheptane and diethyldecane.
Recommended video:
What is a constitutional isomer?
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution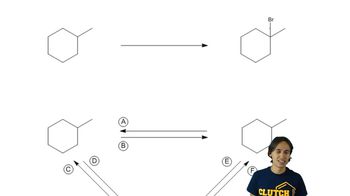


 6:42m
6:42m