Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Structures
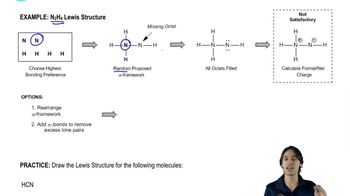
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They help visualize the arrangement of electrons and the connectivity of atoms, which is crucial for understanding molecular geometry and reactivity. In organic chemistry, drawing accurate Lewis structures is essential for predicting the behavior of molecules in chemical reactions.
Recommended video:
Drawing the Lewis Structure for N2H4.
Formal Charge
Formal charge is a concept used to determine the charge of an atom in a molecule based on its valence electrons and the electrons it shares in bonds. It is calculated by taking the number of valence electrons, subtracting the number of non-bonding electrons, and half the number of bonding electrons. Minimizing formal charges in a Lewis structure leads to a more stable and favorable molecular configuration.
Recommended video:
Calculating formal and net charge.
Electron Pair Movement
Electron pair movement refers to the process of shifting electron pairs in a Lewis structure to achieve a more stable arrangement of atoms and minimize formal charges. This can involve forming new bonds or breaking existing ones, which alters the connectivity of the molecule. Understanding how to effectively move electron pairs is crucial for optimizing Lewis structures and predicting molecular behavior.
Recommended video: