Here are the essential concepts you must grasp in order to answer the question correctly.
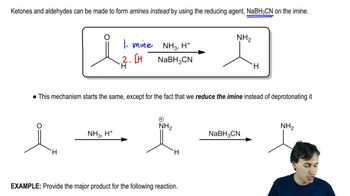
Reduction Reactions
Reduction reactions involve the gain of electrons or the decrease in oxidation state by a molecule. In organic chemistry, this often refers to the conversion of carbonyl compounds (like aldehydes and ketones) to alcohols. The reaction shown in the image uses lithium aluminum hydride (LiAlH4), a strong reducing agent, to reduce a carboxylic acid to an alcohol, which is a key step in synthesizing various organic compounds.
Recommended video:
Formation of Acid Chlorides
Acid chlorides are derived from carboxylic acids by replacing the hydroxyl (-OH) group with a chlorine atom. This transformation is typically achieved using reagents like thionyl chloride (SOCl2) in the presence of a base such as pyridine. Acid chlorides are highly reactive intermediates in organic synthesis, allowing for further transformations into other functional groups, as illustrated in the reaction scheme.
Recommended video:
Acid Chloride Nomenclature
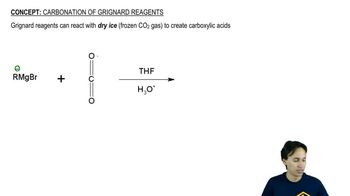
Grignard Reagents
Grignard reagents are organomagnesium compounds that are highly reactive and used to form carbon-carbon bonds in organic synthesis. They are generated by reacting alkyl or aryl halides with magnesium metal. In the reaction scheme, the Grignard reagent (C4H9Li) reacts with an electrophile to form a new carbon-carbon bond, demonstrating their utility in building complex organic molecules.
Recommended video:
Carbonation of Grignard Reagents