Here are the essential concepts you must grasp in order to answer the question correctly.
Lambda Max (λmax)
Lambda max (λmax) refers to the wavelength at which a compound exhibits maximum absorbance in UV-Vis spectroscopy. It is influenced by the electronic structure of the molecule, particularly the presence of conjugated systems, which allow for greater delocalization of π electrons. Compounds with extended conjugation typically show higher λmax values due to lower energy gaps between the ground and excited states.
Recommended video:
The UV-Vis Spectroscopy Concept 3
Conjugation
Conjugation is the overlap of p-orbitals across adjacent double bonds or lone pairs, leading to a delocalization of π electrons. This delocalization stabilizes the molecule and lowers the energy required for electronic transitions, resulting in a higher λmax. The more extensive the conjugated system, the greater the shift in λmax towards longer wavelengths (red shift).
Recommended video:
Substituent Effects
Substituents on a benzene ring can significantly influence the electronic properties of the compound, affecting its λmax. Electron-donating groups (EDGs) can increase electron density in the conjugated system, leading to a higher λmax, while electron-withdrawing groups (EWGs) can decrease electron density, resulting in a lower λmax. Understanding these effects is crucial for predicting the order of λmax in different compounds.
Recommended video:
Directing Effects in Substituted Pyrroles, Furans, and Thiophenes Concept 1

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution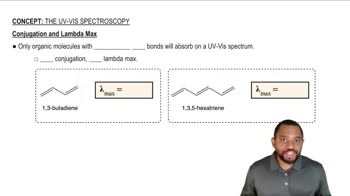



 2:35m
2:35m