Here are the essential concepts you must grasp in order to answer the question correctly.
Infrared Spectroscopy (IR)
Infrared spectroscopy is a technique used to identify functional groups in organic compounds by measuring the absorption of infrared light. Different bonds absorb specific wavelengths of IR radiation, resulting in a spectrum that provides information about the molecular structure. Peaks in the IR spectrum correspond to the vibrational transitions of bonds, allowing chemists to deduce the presence of certain functional groups.
Recommended video:
General Features of IR Spect
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. Common functional groups include hydroxyl (-OH), carbonyl (C=O), and carboxyl (-COOH). Identifying these groups in a compound is crucial for predicting its reactivity and properties, and they are often indicated by distinct peaks in an IR spectrum.
Recommended video:
Identifying Functional Groups
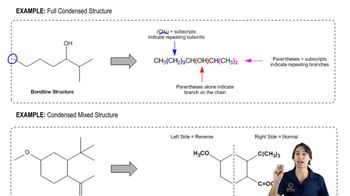
Molecular Structure Interpretation
Interpreting molecular structure involves analyzing the connectivity and arrangement of atoms within a molecule. This includes understanding how different functional groups influence the overall shape and reactivity of the compound. In the context of IR spectroscopy, interpreting the spectrum helps in deducing the molecular structure by correlating observed peaks with specific functional groups and their expected vibrational modes.
Recommended video:
How to interpret condensed structures.
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution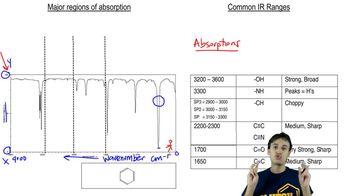


 12:6m
12:6m