Here are the essential concepts you must grasp in order to answer the question correctly.
Wolff–Kishner Reduction
The Wolff–Kishner reduction is a chemical reaction used to convert carbonyl compounds, such as ketones and aldehydes, into alkanes. This process involves the formation of a hydrazone intermediate, which is then treated with a strong base, typically hydrazine and potassium hydroxide, to facilitate the removal of nitrogen gas and reduce the carbonyl compound to an alkane.
Recommended video:
Formation of Hydrazone
The formation of a hydrazone is a key step in the Wolff–Kishner reduction, where a carbonyl compound reacts with hydrazine. This reaction involves the nucleophilic attack of the hydrazine on the carbonyl carbon, leading to the formation of a C=N bond. The resulting hydrazone is a stable intermediate that is crucial for the subsequent reduction step.
Recommended video:
Base-Catalyzed Reduction
In the Wolff–Kishner reduction, the base-catalyzed reduction involves treating the hydrazone with a strong base, which promotes the elimination of nitrogen gas. This step typically occurs through a series of deprotonation and rearrangement reactions, ultimately leading to the formation of the corresponding alkane. The evolution of nitrogen gas is a driving force that helps push the reaction to completion.
Recommended video:
 Verified Solution
Verified Solution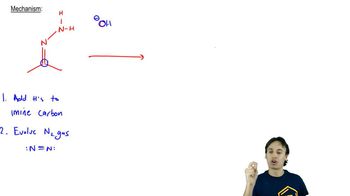


 2:26m
2:26m
