Here are the essential concepts you must grasp in order to answer the question correctly.
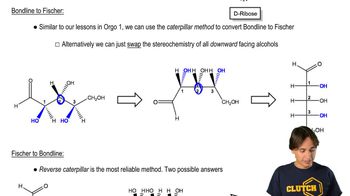
Line-Angle Drawings
Line-angle drawings are a simplified way of representing organic molecules where lines represent bonds between atoms, and vertices represent carbon atoms. This notation allows chemists to visualize complex structures without drawing every atom explicitly. Understanding how to interpret these drawings is crucial for converting them into other representations, such as Fischer projections.
Recommended video:
Fischer Projections
Fischer projections are a two-dimensional representation of three-dimensional organic molecules, particularly useful for depicting stereochemistry. In this format, vertical lines represent bonds that project behind the plane of the page, while horizontal lines represent bonds that project out towards the viewer. Mastery of Fischer projections is essential for accurately conveying the spatial arrangement of substituents around chiral centers.
Recommended video:
Monosaccharides - Drawing Fischer Projections
Stereochemistry and Bond Rotation
Stereochemistry involves the study of the spatial arrangement of atoms in molecules and how this affects their chemical behavior. Bond rotation refers to the ability to rotate around single bonds, which can change the conformation of a molecule. Understanding how to manipulate bond rotations is key when converting line-angle drawings to Fischer projections, as it ensures that the correct stereochemical relationships are maintained.
Recommended video:
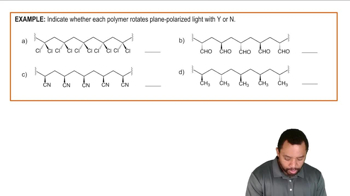
Polymer Stereochemistry Example 1