Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Ion
A molecular ion is a charged species formed when a molecule loses or gains an electron. In mass spectrometry, the molecular ion corresponds to the intact molecule's mass, represented by its m/z (mass-to-charge) ratio. The m/z value of 86 indicates that the molecular ion has a mass of 86 daltons, which is crucial for deducing possible molecular formulas.
Recommended video:
Metal Ion Catalysis Concept 1
Empirical Formula
The empirical formula represents the simplest whole-number ratio of atoms in a compound. It provides a foundational understanding of the compound's composition, which can help in deducing possible molecular formulas. For a molecular ion with an m/z of 86, the empirical formula can guide the identification of potential combinations of elements that yield this mass.
Recommended video:
How to use IHD with molecular formula.
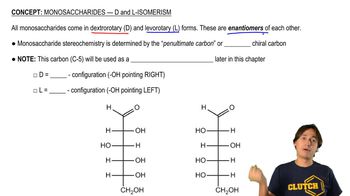
Isomerism
Isomerism refers to the existence of compounds with the same molecular formula but different structural arrangements or spatial orientations. For a molecular ion with an m/z of 86, various isomers can be proposed, each with distinct properties and reactivities. Understanding isomerism is essential for exploring the diversity of compounds that can correspond to the same molecular ion mass.
Recommended video:
Monosaccharides - D and L Isomerism
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:48m
1:48m