Here are the essential concepts you must grasp in order to answer the question correctly.
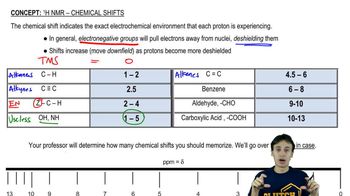
Chemical Shift (δ)
Chemical shift, denoted as δ, refers to the resonance frequency of a nucleus relative to a standard reference frequency. It is measured in parts per million (ppm) and provides insight into the electronic environment surrounding the hydrogen atom. In this case, a δ value of 5.34 indicates that the hydrogen is likely in a deshielded environment, often associated with electronegative atoms or unsaturation.
Recommended video:
Coupling Constants (J)
Coupling constants, represented as J, quantify the interaction between nuclear spins of neighboring atoms, influencing the splitting patterns observed in NMR spectra. The values Jₐ꜀ = 12 Hz and Jₐ₆ = 2 Hz indicate the strength of the coupling between the hydrogen atom in question and its neighboring protons. These constants help determine the number of peaks and their relative intensities in the NMR signal.
Recommended video:
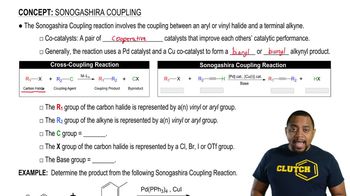
Sonogashira Coupling Reaction
NMR Signal Splitting
NMR signal splitting occurs when a hydrogen atom is coupled to neighboring hydrogen atoms, resulting in multiple peaks in the spectrum. The number of peaks is determined by the n+1 rule, where n is the number of equivalent neighboring protons. In this scenario, the coupling constants suggest that the hydrogen will exhibit a complex splitting pattern, reflecting its interactions with adjacent protons.
Recommended video:
General Assumption for 1H NMR Signals