Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Catalyzed Reactions
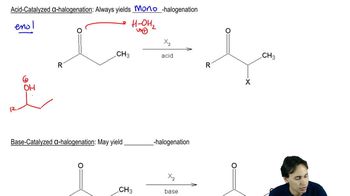
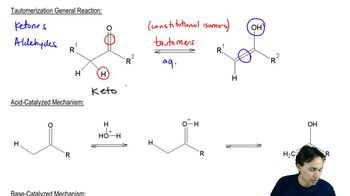
Acid-catalyzed reactions involve the use of an acid to increase the rate of a chemical reaction. In organic chemistry, acids can protonate substrates, making them more electrophilic and susceptible to nucleophilic attack. This mechanism is crucial in reactions where the formation of a more reactive intermediate is necessary, such as in the bromination of ketones.
Recommended video:
Bromination Mechanism
Bromination is the process of adding bromine to an organic compound. In the case of ketones like pentan-3-one, the mechanism typically involves the formation of a bromonium ion or a similar electrophilic species. Understanding how bromine interacts with the carbonyl group and the subsequent steps leading to product formation is essential for proposing a detailed mechanism.
Recommended video:
Mechanism of Allylic Bromination.
Keto-Enol Tautomerism
Keto-enol tautomerism is the equilibrium between a keto form (a carbonyl compound) and its corresponding enol form (an alcohol with a double bond). In the context of pentan-3-one, the enol form can be more reactive towards electrophiles like bromine. Recognizing this equilibrium is important for understanding how the bromination can occur at different sites on the molecule.
Recommended video:
Tautomerization Mechanisms