Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a powerful analytical technique used to determine the structure of organic compounds. It works by applying a magnetic field to nuclei of certain isotopes, such as hydrogen, causing them to resonate at specific frequencies. The resulting spectrum provides information about the number of hydrogen atoms, their environment, and how they are connected, which is crucial for identifying the structure of the compound formed in the reaction.
Recommended video:
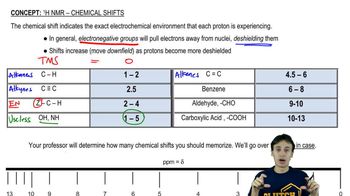
Chemical Shifts
Chemical shifts in an NMR spectrum indicate the electronic environment surrounding hydrogen atoms in a molecule. Measured in parts per million (ppm), these shifts help differentiate between protons in various chemical environments, such as those adjacent to electronegative atoms or in aliphatic versus aromatic systems. Understanding chemical shifts is essential for interpreting the NMR data and assigning peaks to specific protons in the structure of the product.
Recommended video:
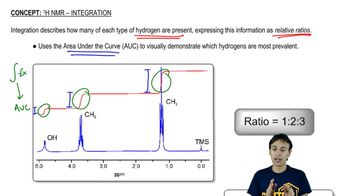
Integration of Peaks
The integration of peaks in an NMR spectrum reflects the relative number of hydrogen atoms contributing to each signal. The area under each peak corresponds to the number of protons in that environment, allowing chemists to deduce the ratio of different types of protons in the molecule. In this case, the spectrum shows peaks for 4 H and 2 H, which can help identify the structure of the impurities formed during the reaction by correlating these values with the expected proton counts in potential structures.
Recommended video:

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: