Here are the essential concepts you must grasp in order to answer the question correctly.
Amino Acid Structure
Amino acids are organic compounds that serve as the building blocks of proteins. Each amino acid has a central carbon atom bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a variable R group that determines its unique properties. Understanding the structure of amino acids is essential for predicting their behavior in different pH environments.
Recommended video:
Zwitterion Formation
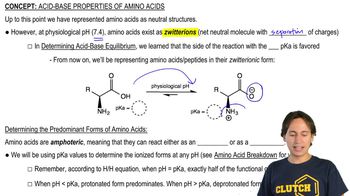
At certain pH levels, amino acids can exist as zwitterions, which are molecules that have both positive and negative charges but are overall neutral. This occurs when the amino group is protonated (-NH3+) and the carboxyl group is deprotonated (-COO-). The formation of zwitterions is crucial for understanding the predominant forms of amino acids in solution, especially at varying pH levels.
Recommended video:
Why Amino Acids Exist as Zwitterions
pH and Amino Acid Ionization
The pH of a solution affects the ionization state of amino acids. At low pH (like pH 2), amino acids tend to be protonated, leading to a higher concentration of positively charged species. This concept is vital for predicting the predominant forms of alanine, lysine, and aspartic acid in acidic conditions, as their side chains will also influence their overall charge and structure.
Recommended video:
The 7 Ionizable Amino Acids
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution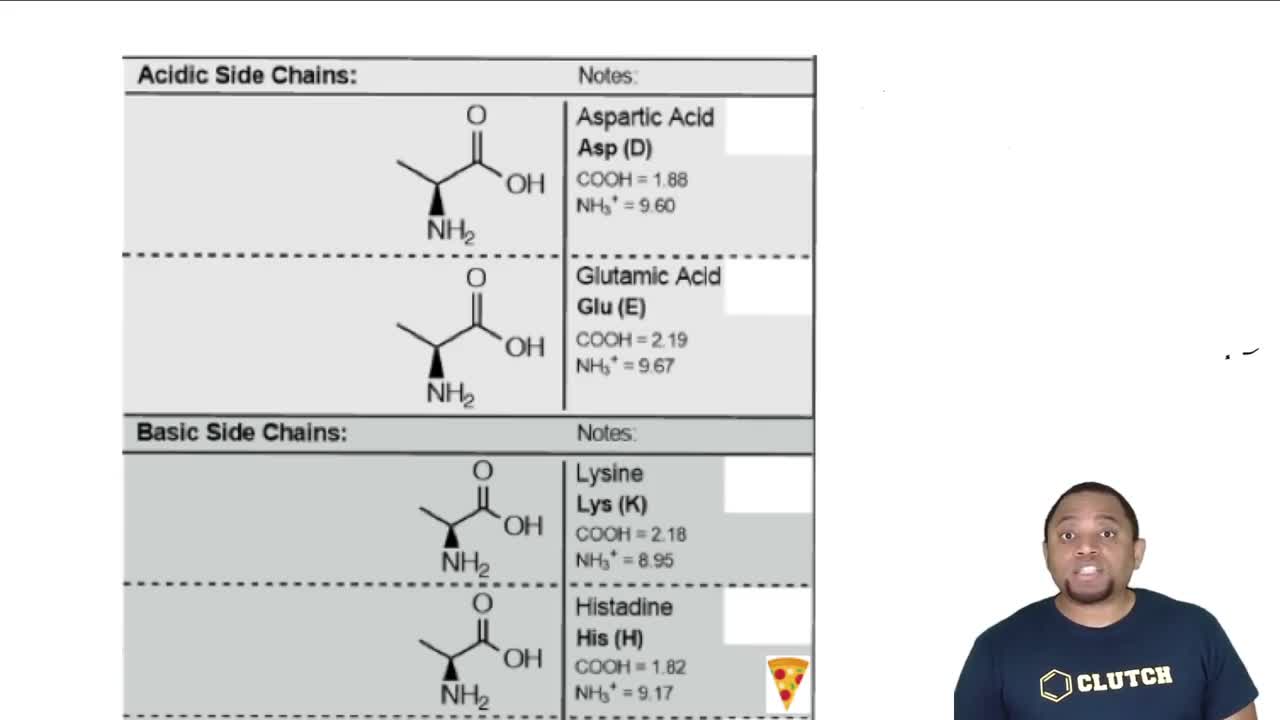


 4:41m
4:41m