Here are the essential concepts you must grasp in order to answer the question correctly.
Isoelectric Point (pI)
The isoelectric point (pI) is the pH at which a molecule, such as an amino acid, carries no net electrical charge. For phenylalanine, this occurs at pH 5.5, meaning that at this pH, the positive and negative charges on the molecule balance each other out. Understanding pI is crucial for predicting the behavior of amino acids in different pH environments.
Recommended video:
Definition of Isoelectric Point
Amino Acid Structure
Amino acids have a general structure consisting of a central carbon atom, an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a variable side chain (R group). For phenylalanine, the side chain is a benzyl group, which influences its properties and behavior in solution. Recognizing how these groups interact with pH is essential for drawing the correct structures at different pH levels.
Recommended video:
Protonation and Deprotonation
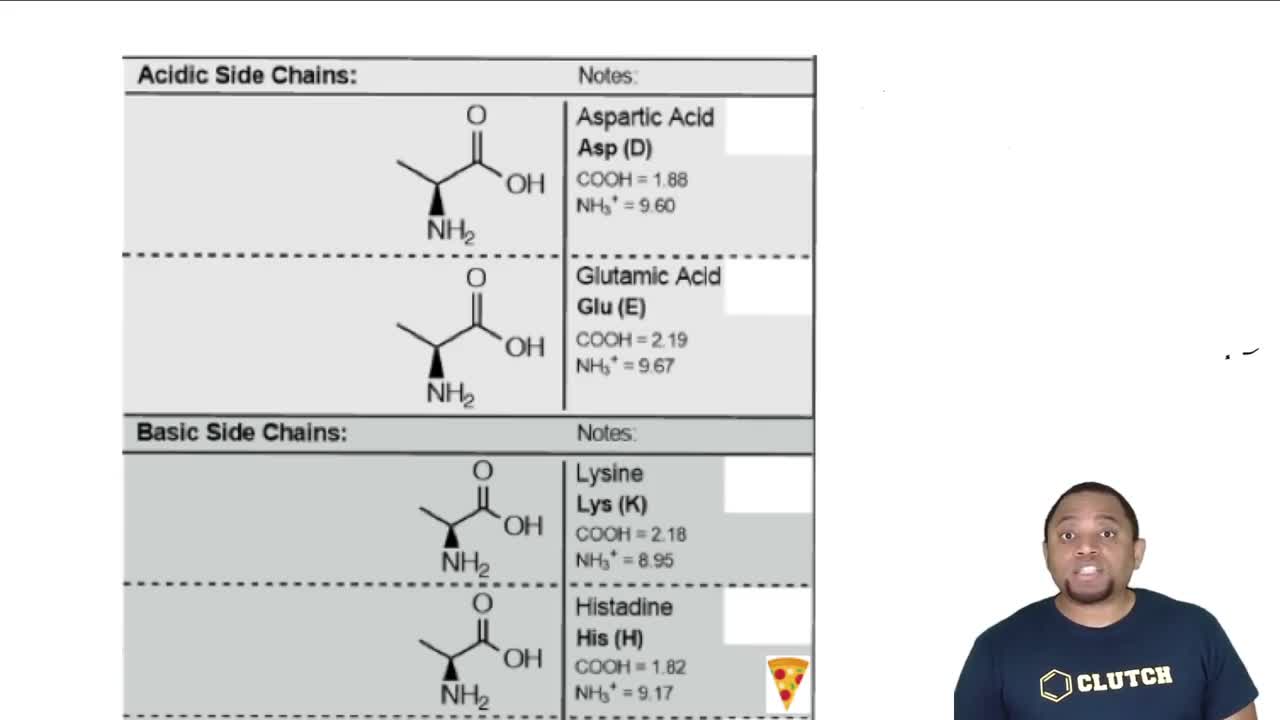
Protonation and deprotonation refer to the addition or removal of protons (H+) from a molecule, affecting its charge and structure. At low pH (1), amino acids are typically protonated, leading to a positive charge. At high pH (11), they are deprotonated, resulting in a negative charge. Understanding these processes is vital for determining the predominant form of phenylalanine at various pH levels.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: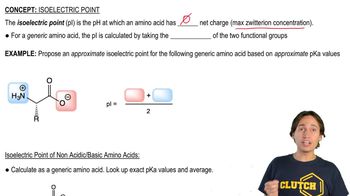


 3:19m
3:19m