Here are the essential concepts you must grasp in order to answer the question correctly.
Claisen Condensation
The Claisen condensation is a reaction between two esters or an ester and a carbonyl compound in the presence of a strong base, typically sodium ethoxide. This reaction results in the formation of a β-keto ester or a β-diketone. Understanding this mechanism is crucial for predicting the products, as it involves nucleophilic attack and subsequent elimination of an alcohol.
Recommended video:
Nucleophilic Acyl Substitution
Nucleophilic acyl substitution is a fundamental reaction in organic chemistry where a nucleophile attacks the carbonyl carbon of an acyl compound, leading to the substitution of a leaving group. In the context of Claisen reactions, the nucleophile is often an enolate ion formed from an ester, which plays a key role in forming the new carbon-carbon bond and determining the final product.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
Enolate Ion Formation
Enolate ions are reactive intermediates formed when a base abstracts a proton from the α-carbon of a carbonyl compound, resulting in a resonance-stabilized anion. In Claisen reactions, the formation of the enolate is essential as it acts as the nucleophile that attacks another carbonyl compound, facilitating the condensation process and influencing the product's structure.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution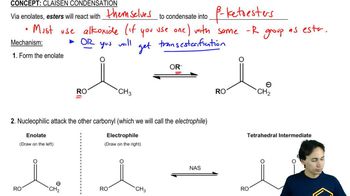



 6:08m
6:08m