Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation Reactions
Oxidation reactions involve the loss of electrons or an increase in oxidation state by a molecule, atom, or ion. In organic chemistry, these reactions often lead to the formation of carbonyl compounds or carboxylic acids from alcohols or alkenes. The reaction of organic compounds with oxidizing agents like H2CrO4 (chromic acid) typically results in the oxidation of side chains, particularly in aromatic compounds.
Recommended video:
Chromic Acid (H2CrO4)
Chromic acid is a strong oxidizing agent commonly used in organic synthesis to oxidize alcohols and alkenes. It can convert primary alcohols to carboxylic acids and secondary alcohols to ketones. In the context of the provided reaction, H2CrO4 is used to oxidize the alkyl side chain of the aromatic compound, leading to the formation of a carboxylic acid or other oxidized products depending on the structure of the side chain.
Recommended video:
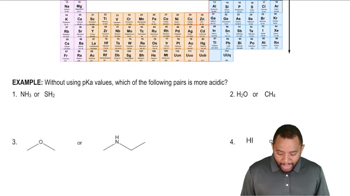
Using factors affecting acidity to rank acids
Aromatic Compounds and Side-Chain Oxidation
Aromatic compounds are characterized by their stable ring structure and delocalized π-electrons. Side-chain oxidation refers to the process where functional groups attached to the aromatic ring are oxidized, often resulting in the formation of carboxylic acids or ketones. In the given reaction, the alkyl side chain of the aromatic compound is oxidized by H2CrO4, which can significantly alter the compound's reactivity and properties.
Recommended video:

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 3:20m
3:20m