In this video, we're going to introduce osmosis. And so osmosis is a type of passive diffusion, which means that absolutely no energy is required for osmosis to occur. Now, osmosis is defined as the passive diffusion of a solvent across a semipermeable membrane. Now recall that solvents are substances that dissolve other substances, and usually in biology the solvent is going to be water. And so really we can define osmosis as the passive diffusion of water across a semi permeable membrane such as a biological membrane or a cell membrane. Now really the direction that water will flow across that semipermeable membrane or biological membrane will depend on the tonicity of the solution. And so the tonicity is defined as the relative concentration of solute dissolved in the solutions. And when it comes to tonicity, really there are 3 terms that you all should know and we have these three terms down below. And what you'll notice about all three of these terms is that they all end in tonic, and tonic is just referring to the tenacity. And so, what you'll notice is the difference between these three terms is going to be the prefix that comes right before, and so that's why we have the prefix as interactive. And so the first term that you all need to know when it comes to tonicity is the term hypotonic. Now hypo is a prefix that means low and it even rhymes with low, hypolo. And so hypotonic solutions are going to have lower solute concentrations. Now the second term that you all should know is isotonic. And iso is a prefix that means equal. And so isotonic solutions are going to have an equal solute concentration. The 3rd and final term that you need to know when it comes to tonicity is hypertonic solution. And hyper kind of sounds like hypo, but hypo round rhymes with low, but hyper does not rhyme with low. So hyper is actually going to mean higher solute concentrations. And so what you'll notice is that lower, equal, and higher are all words of comparison. Something can't be lower unless it's lower than something else. Something can't be equal unless it's equal to something else, and something can't be higher unless it's higher than something else. And so essentially what we're saying is that lower, equal, and higher are words of comparison, you need to be comparing at least 2 regions and the same goes with hypo, iso and hypertonic. You need to be using these words when you're comparing 2 regions. Now the 2 regions that we're typically going to be comparing are the inside of the cell, that's region number 1, and the second region that we'll be comparing is the region on the outside of the cell or the surrounding solution outside of the cell. So down below in our example, notice that it's asking us to label the tenacity of the outside solution on the outside of the cell with respect to the solution on the inside of the cell. So we need to be comparing the outside solution on the outside of the cell with the inside of the cell, which would be over here, and we'll be doing that in each of these three scenarios down below. So when we take a look at this first scenario over here on the left hand side, notice that there are these green solutes, and there are only 2 green solutes on the outside. Whereas on the inside, there's a much higher concentration of these green solutes. And so because the outside has a lower solute concentration than the inside, we can label the outside solution. We're labeling the outside solution here and the outside is lower, so it's going to be hypotonic. The outside solution will be hypotonic because it has a lower solute concentration than the inside of the cell, which has a higher solute concentration. Remember, the solutes here are the green circles. Now moving on to this scenario here in the middle, again we're gonna be labeling the outside solution. And what you'll notice is that the outside solution has a concentration that's pretty much equal to the concentration of solute on the inside. And so because the outside solution and inside solution have equal solute concentrations, that means that the outside solution will be isotonic with respect to the inside. So in here, in the middle, we can label this as isotonic. The outside is isotonic with respect to the inside. And then notice over here in the final, image over here on the right hand side, once again, we're labeling the outside solution, and notice that the outside solution has a much higher concentration than the inside. And so, that means that the outside, because it's higher, it's going to be hypertonic. And so we can label the outside solution hypertonic here, and that will be the outside solution once again because it has a higher concentration of solute than the inside. And so now what we can do now that we've labeled the outside solution with all 3 of these terms, and remember the outside solution is gonna be here in all three cases, we can now try to label the inside solutions for each of these. And so, when you take a look at the inside of these, notice that the inside here has a higher concentration with respect to the outside, which has a lower concentration. So the inside of the cell here, because it has a higher solute concentration, it's going to be hypertonic. So we can label the inside here as hyper. I'm just gonna put hyper here, just to save some space, but it would be hypertonic. So now let's label the inside over here. Well, the inside here has an equal solute concentration with respect to the outside. So that means that the inside is also going to be isotonic, but I'll just put iso here just for short. And then over here, labeling the inside solution, notice that the inside here has a lower solute concentration than the outside, which has a higher solute concentration. And if it had if the inside has a lower solute concentration, that means it will be hypotonic on the inside. And so here, we can label the inside as hypotonic. And so you can see that these words are words of comparison once again. You must be comparing 2 regions, and usually those regions are going to be the outside of the cell with the inside of the cell. And so this here concludes our introduction to osmosis and as we move forward we'll be able to continue to talk more and more about now, that we know about the tonicity we can talk about, the direction of water flow. So we'll talk more about that in our next video and I'll see you guys there.
- 1. Introduction to Microbiology3h 21m
- Introduction to Microbiology16m
- Introduction to Taxonomy26m
- Scientific Naming of Organisms9m
- Members of the Bacterial World10m
- Introduction to Bacteria9m
- Introduction to Archaea10m
- Introduction to Eukarya20m
- Acellular Infectious Agents: Viruses, Viroids & Prions19m
- Importance of Microorganisms20m
- Scientific Method27m
- Experimental Design30m
- 2. Disproving Spontaneous Generation1h 18m
- 3. Chemical Principles of Microbiology3h 38m
- 4. Water1h 28m
- 5. Molecules of Microbiology2h 23m
- 6. Cell Membrane & Transport3h 28m
- Cell Envelope & Biological Membranes12m
- Bacterial & Eukaryotic Cell Membranes8m
- Archaeal Cell Membranes18m
- Types of Membrane Proteins8m
- Concentration Gradients and Diffusion9m
- Introduction to Membrane Transport14m
- Passive vs. Active Transport13m
- Osmosis33m
- Simple and Facilitated Diffusion17m
- Active Transport30m
- ABC Transporters11m
- Group Translocation7m
- Types of Small Molecule Transport Review9m
- Endocytosis and Exocytosis15m
- 7. Prokaryotic Cell Structures & Functions5h 52m
- Prokaryotic & Eukaryotic Cells26m
- Binary Fission11m
- Generation Times16m
- Bacterial Cell Morphology & Arrangements35m
- Overview of Prokaryotic Cell Structure10m
- Introduction to Bacterial Cell Walls26m
- Gram-Positive Cell Walls11m
- Gram-Negative Cell Walls20m
- Gram-Positive vs. Gram-Negative Cell Walls11m
- The Glycocalyx: Capsules & Slime Layers12m
- Introduction to Biofilms6m
- Pili18m
- Fimbriae & Hami7m
- Introduction to Prokaryotic Flagella12m
- Prokaryotic Flagellar Structure18m
- Prokaryotic Flagellar Movement11m
- Proton Motive Force Drives Flagellar Motility5m
- Chemotaxis14m
- Review of Prokaryotic Surface Structures8m
- Prokaryotic Ribosomes16m
- Introduction to Bacterial Plasmids13m
- Cell Inclusions9m
- Endospores16m
- Sporulation5m
- Germination5m
- 8. Eukaryotic Cell Structures & Functions2h 18m
- 9. Microscopes2h 46m
- Introduction to Microscopes8m
- Magnification, Resolution, & Contrast10m
- Introduction to Light Microscopy5m
- Light Microscopy: Bright-Field Microscopes23m
- Light Microscopes that Increase Contrast16m
- Light Microscopes that Detect Fluorescence16m
- Electron Microscopes14m
- Reviewing the Different Types of Microscopes10m
- Introduction to Staining5m
- Simple Staining14m
- Differential Staining6m
- Other Types of Staining11m
- Reviewing the Types of Staining8m
- Gram Stain13m
- 10. Dynamics of Microbial Growth4h 36m
- Biofilms16m
- Growing a Pure Culture5m
- Microbial Growth Curves in a Closed System21m
- Temperature Requirements for Microbial Growth18m
- Oxygen Requirements for Microbial Growth22m
- pH Requirements for Microbial Growth8m
- Osmolarity Factors for Microbial Growth14m
- Reviewing the Environmental Factors of Microbial Growth12m
- Nutritional Factors of Microbial Growth30m
- Growth Factors4m
- Introduction to Cultivating Microbial Growth5m
- Types of Solid Culture Media4m
- Plating Methods16m
- Measuring Growth by Direct Cell Counts9m
- Measuring Growth by Plate Counts14m
- Measuring Growth by Membrane Filtration6m
- Measuring Growth by Biomass15m
- Introduction to the Types of Culture Media5m
- Chemically Defined Media3m
- Complex Media4m
- Selective Media5m
- Differential Media9m
- Reducing Media4m
- Enrichment Media7m
- Reviewing the Types of Culture Media8m
- 11. Controlling Microbial Growth4h 10m
- Introduction to Controlling Microbial Growth29m
- Selecting a Method to Control Microbial Growth44m
- Physical Methods to Control Microbial Growth49m
- Review of Physical Methods to Control Microbial Growth7m
- Chemical Methods to Control Microbial Growth16m
- Chemicals Used to Control Microbial Growth6m
- Liquid Chemicals: Alcohols, Aldehydes, & Biguanides15m
- Liquid Chemicals: Halogens12m
- Liquid Chemicals: Surface-Active Agents17m
- Other Types of Liquid Chemicals14m
- Chemical Gases: Ethylene Oxide, Ozone, & Formaldehyde13m
- Review of Chemicals Used to Control Microbial Growth11m
- Chemical Preservation of Perishable Products10m
- 12. Microbial Metabolism5h 16m
- Introduction to Energy15m
- Laws of Thermodynamics15m
- Chemical Reactions9m
- ATP20m
- Enzymes14m
- Enzyme Activation Energy9m
- Enzyme Binding Factors9m
- Enzyme Inhibition10m
- Introduction to Metabolism8m
- Negative & Positive Feedback7m
- Redox Reactions22m
- Introduction to Aerobic Cellular Respiration25m
- Types of Phosphorylation12m
- Glycolysis19m
- Entner-Doudoroff Pathway11m
- Pentose-Phosphate Pathway10m
- Pyruvate Oxidation8m
- Krebs Cycle16m
- Electron Transport Chain19m
- Chemiosmosis7m
- Review of Aerobic Cellular Respiration19m
- Fermentation & Anaerobic Respiration23m
- 13. Photosynthesis2h 31m
- 14. DNA Replication2h 25m
- 15. Central Dogma & Gene Regulation7h 14m
- Central Dogma7m
- Introduction to Transcription20m
- Steps of Transcription22m
- Transcription Termination in Prokaryotes7m
- Eukaryotic RNA Processing and Splicing20m
- Introduction to Types of RNA9m
- Genetic Code25m
- Introduction to Translation30m
- Steps of Translation23m
- Review of Transcription vs. Translation12m
- Prokaryotic Gene Expression21m
- Review of Prokaryotic vs. Eukaryotic Gene Expression13m
- Introduction to Regulation of Gene Expression13m
- Prokaryotic Gene Regulation via Operons27m
- The Lac Operon21m
- Glucose's Impact on Lac Operon25m
- The Trp Operon20m
- Review of the Lac Operon & Trp Operon11m
- Introduction to Eukaryotic Gene Regulation9m
- Eukaryotic Chromatin Modifications16m
- Eukaryotic Transcriptional Control22m
- Eukaryotic Post-Transcriptional Regulation28m
- Post-Translational Modification6m
- Eukaryotic Post-Translational Regulation13m
- 16. Microbial Genetics4h 44m
- Introduction to Microbial Genetics11m
- Introduction to Mutations20m
- Methods of Inducing Mutations15m
- Prototrophs vs. Auxotrophs13m
- Mutant Detection25m
- The Ames Test14m
- Introduction to DNA Repair5m
- DNA Repair Mechanisms37m
- Horizontal Gene Transfer18m
- Bacterial Transformation11m
- Transduction32m
- Introduction to Conjugation6m
- Conjugation: F Plasmids18m
- Conjugation: Hfr & F' Cells19m
- Genome Variability21m
- CRISPR CAS11m
- 17. Biotechnology3h 0m
- 18. Viruses, Viroids, & Prions4h 56m
- Introduction to Viruses20m
- Introduction to Bacteriophage Infections14m
- Bacteriophage: Lytic Phage Infections12m
- Bacteriophage: Lysogenic Phage Infections17m
- Bacteriophage: Filamentous Phage Infections8m
- Plaque Assays9m
- Introduction to Animal Virus Infections10m
- Animal Viruses: 1. Attachment to the Host Cell7m
- Animal Viruses: 2. Entry & Uncoating in the Host Cell19m
- Animal Viruses: 3. Synthesis & Replication22m
- Animal Viruses: DNA Virus Synthesis & Replication14m
- Animal Viruses: RNA Virus Synthesis & Replication22m
- Animal Viruses: Antigenic Drift vs. Antigenic Shift9m
- Animal Viruses: Reverse-Transcribing Virus Synthesis & Replication9m
- Animal Viruses: 4. Assembly Inside Host Cell8m
- Animal Viruses: 5. Release from Host Cell15m
- Acute vs. Persistent Viral Infections25m
- COVID-19 (SARS-CoV-2)14m
- Plant Viruses12m
- Viroids6m
- Prions13m
- 19. Innate Immunity7h 15m
- Introduction to Immunity8m
- Introduction to Innate Immunity17m
- Introduction to First-Line Defenses5m
- Physical Barriers in First-Line Defenses: Skin13m
- Physical Barriers in First-Line Defenses: Mucous Membrane9m
- First-Line Defenses: Chemical Barriers24m
- First-Line Defenses: Normal Microflora5m
- Introduction to Cells of the Immune System15m
- Cells of the Immune System: Granulocytes29m
- Cells of the Immune System: Agranulocytes25m
- Introduction to Cell Communication5m
- Cell Communication: Surface Receptors & Adhesion Molecules16m
- Cell Communication: Cytokines27m
- Pattern Recognition Receptors (PRRs)45m
- Introduction to the Complement System24m
- Activation Pathways of the Complement System23m
- Effects of the Complement System23m
- Review of the Complement System12m
- Phagoctytosis21m
- Introduction to Inflammation18m
- Steps of the Inflammatory Response26m
- Fever8m
- Interferon Response25m
- 20. Adaptive Immunity7h 14m
- Introduction to Adaptive Immunity32m
- Antigens12m
- Introduction to T Lymphocytes38m
- Major Histocompatibility Complex Molecules20m
- Activation of T Lymphocytes21m
- Functions of T Lymphocytes25m
- Review of Cytotoxic vs Helper T Cells13m
- Introduction to B Lymphocytes27m
- Antibodies14m
- Classes of Antibodies35m
- Outcomes of Antibody Binding to Antigen15m
- T Dependent & T Independent Antigens21m
- Clonal Selection20m
- Antibody Class Switching17m
- Affinity Maturation14m
- Primary and Secondary Response of Adaptive Immunity21m
- Immune Tolerance28m
- Regulatory T Cells10m
- Natural Killer Cells16m
- Review of Adaptive Immunity25m
- 21. Principles of Disease6h 57m
- Symbiotic Relationships12m
- The Human Microbiome46m
- Characteristics of Infectious Disease47m
- Stages of Infectious Disease Progression26m
- Koch's Postulates26m
- Molecular Koch's Postulates11m
- Bacterial Pathogenesis36m
- Introduction to Pathogenic Toxins6m
- Exotoxins Cause Damage to the Host40m
- Endotoxin Causes Damage to the Host13m
- Exotoxins vs. Endotoxin Review13m
- Immune Response Damage to the Host15m
- Introduction to Avoiding Host Defense Mechanisms8m
- 1) Hide Within Host Cells5m
- 2) Avoiding Phagocytosis31m
- 3) Surviving Inside Phagocytic Cells10m
- 4) Avoiding Complement System9m
- 5) Avoiding Antibodies25m
- Viruses Evade the Immune Response27m
Osmosis - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIOsmosis is the passive diffusion of water across a semipermeable membrane, influenced by the tonicity of surrounding solutions. Solutions can be hypotonic (lower solute concentration), isotonic (equal solute concentration), or hypertonic (higher solute concentration). Water moves from hypotonic to hypertonic solutions to achieve equilibrium. In hypotonic environments, animal cells may lyse, while plant cells benefit from increased turgor pressure. Isotonic conditions maintain cell size, preferred by animal cells, whereas hypertonic environments lead to dehydration and cell shrinkage for both plant and animal cells.
Osmosis
Video transcript
Osmosis Example 1
Video transcript
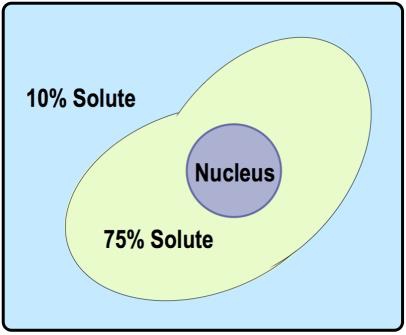
Alright. So here we have an example problem that's asking, what is the tenacity of the outside solution in comparison to the inside of the cell? And we've got these 4 potential answer options down below. Now, of course, when we look at option d, it says electrotonic, which is not a word that we described in our last lesson video. So for sure, we know that we can eliminate answer option d. But hypotonic, isotonic, and hypertonic are all terms that we talked about in our last lesson video. So it's going to be between one of these three answers.
And so when we take a look at this image down below, what we need to notice is that there is a beaker, and the beaker has an outside solution here, and then it has a cell, a red blood cell right here in the middle. This image is indicating that the outside solution is 10% solute and it indicates that inside of the red blood cell is 0.1% solute. So what we need to realize is that 10% and 0.1%, when we compare these two numbers, 10% is higher. It is a higher solute concentration, and 0.1% solute is lower.
Recall from our last lesson video that isotonic is a word that means equal solute concentrations. But because 10% solute is higher than 0.1% solute, they are not equal to each other. 10% is not equal to 0.1%. So we know that we can eliminate answer option b, isotonic. It would be isotonic if they both had the exact same percentage of solute, the same concentration of solute.
What we need to realize here is that this problem is specifically asking us to label the tonicity of the outside solution. So we need to focus on the outside solution. The outside solution is 10% Solute. It is a higher Solute Concentration. And so recall the word that means higher solute concentration is hypertonic. Hypertonic means higher solute concentration, and so that means that the outside solution, the one that we're trying to label here, is going to be hypertonic with respect to the inside of the cell. Thus, the correct answer here is going to be answer option c.
This, of course, means that the inside of the red blood cell is going to be hypotonic. So inside here, we can say it is hypotonic, and the outside here, we can say is hypertonic. And so, again, we're labeling the outside solution, so it will be hypertonic, and option a is not going to be correct for the outside solution. So, option c, hypertonic, is the correct answer for this example, and I'll see you guys in our next video.
Direction of Osmosis
Video transcript
So now that we've introduced tonicity in our last lesson video, in this video we're going to talk about how tonicity affects the direction of osmosis. And so what we need to recall from our previous lesson videos is that biological membranes are semi-permeable, which means that some things can get across the membrane, but other things cannot get across the membrane. And so, if the solutes that are in a solution cannot diffuse across the membrane from areas of high concentration to areas of low concentration, then osmosis is going to occur. And recall, osmosis is the diffusion of water across the membrane. And so here's what you guys need to know: Water will always move from hypotonic solutions towards hypertonic solutions. And so this is the direction of water movement, from hypo towards hypertonic solutions. And so this is going to allow water to move towards the more concentrated solution of solute in order to dilute that solution and it will continue to move towards that solution until it becomes isotonic. And so essentially the water is moving to help to try to create equal solute concentrations in both solutions.
Now, you might be thinking, wait a second, doesn't that go against the natural tendency of diffusion? Don't substances always diffuse from high concentration to low concentration? But here you're telling me that it's diffusing from low concentration to high concentration. So how does that make sense? Well, what's important to keep in mind is that hypotonic and hypertonic are terms that refer to the solute concentration. However, water is not the solute, water is the solvent. And so what's important to keep in mind is that water is still moving from areas of higher concentration of water towards lower concentrations of water. So let's take a look at this in a little bit more depth here to clear this up. Hypotonic solutions, they do have lower solute concentrations like we described in our last lesson video. However, hypotonic solutions, even though they have lower solute concentrations, they actually have higher water concentrations. And hypertonic solutions, they do have higher solute concentration just like what we described in our last lesson video. However, hypertonic solutions, although they have higher solute concentrations, they have lower water concentrations. And so what's really important to just realize here is that water is the solvent, whereas solutes are not the solvent. And so, these terms hypo and hyper are referring to the concentrations of solute. However, water is not the concentration of solute and water is still going to be moving from areas of higher concentration of water towards lower concentrations of water. So once again, let's take a look at our image down below to try to clear this up even further. And so notice what we have here down the middle is a biological membrane, which is semi-permeable, meaning that some things can cross the membrane but other things cannot cross the membrane. And so in a scenario where the solutes cannot cross the membrane. Although the solutes would love to diffuse from higher concentration to lower concentration across the membrane, they can't because they're being blocked. And so in some scenarios, if the solutes cannot diffuse across the membrane, instead of the solutes diffusing from high to low concentration, because they can't, what's going to happen is water is going to move instead. And in areas that are hypotonic, yes, they have lower solute concentration because hypotonic is referring to the lower solute concentration, but it turns out that they have actually have a higher water concentration. They have higher water. And hypertonic solutions, yes, they have a higher solute concentration because, remember, hypertonic is referring to the solute concentration being higher. However, higher solute concentration means that it's going to have lower water concentration. And by the way, the brackets that you see here, just mean the concentration of. And so when you see something in brackets like water in brackets, it means concentration of water, and solute in brackets means concentration of solute. And so remember what we said, what you need to know is right here. Water will always move from hypo towards hypertonic solutions. And so over here on the left we have hypo, over here on the right we have hyper. So water is always going to flow in this direction. And so if you're able to remember, water flows from hypo towards hyper, then you'll be good on most of your osmosis questions. And so we'll be able to get some practice applying the concepts that we've learned here, but once again, keep in mind, water always flows from hypo towards hypertonic solutions.
So that being said, I'll see you all in our next video.
Osmosis is best defined as the movement of:
Which direction would you expect water to move across the cell membrane?
Environmental Tonicity Affects Cells
Video transcript
So in our previous lesson videos, we introduced tonicity and the direction of osmosis across a membrane. How water flows from hypotonic solutions towards hypertonic solutions. In this video, we're going to talk about how the environmental tonicity affects cells. We're mainly going to be focusing on the outside environment that surrounds a cell. If the outside environment that surrounds a cell is hypotonic, then in this scenario, water is going to enter the cells and cause the cells to swell up or enlarge like a hippopotamus. You can think that hypotonic environments cause cells to swell up or enlarge like a hippopotamus. For animal cells that do not have a cell wall, they could potentially lyse or burst under these conditions. You can think of it like a balloon. If the cell was a balloon, if you blow too much air into a balloon then it's going to expand, enlarge or swell like a hippopotamus. But if you blow too much air into it, then it could potentially pop open. The same goes for cells, if too much water enters the cells, they could swell up too much. If they swell up too much, they could potentially lyse or burst. For animal cells that do not have cell walls, they do not prefer hypotonic environments because they could potentially lyse or burst, which could kill them. However, for plant cells that do have cell walls, the cell walls can prevent the expansion of the cell membrane. That means that the cell membrane is protected from lysing or bursting open with plant cell walls. Plant cell walls and plant cells actually prefer hypotonic environments, and this is because they do not lyse or burst open, and when the water enters the plant cells it actually increases what is known as turgor pressure. Turgor pressure is pretty much defined as the water pressure that's on the cell membrane, and this allows plants to have their upright and healthy structure.
Let's take a look at our image down below to clear up some of these ideas, and we're gonna be focusing mainly on the left hand side of the image over here. When the outside environment that is surrounding a cell is hypotonic, that could potentially cause a cell to swell up like a hippopotamus. Notice that we're showing you an animal cell and notice that it's a blood cell and water is moving towards the inside of the cell in the hypotonic environment. Notice that when water goes into the cell, just like blowing air into a balloon, the cell is going to expand. But if it expands too much, it could potentially cause cell lysis, essentially causing the cell to burst open, killing the cell. And that's exactly what's happening right here with this animal cell. Animal cells, because they do not have cell walls, they could undergo cell lysis and that could kill the cell, and so animal cells do not prefer hypotonic environments. However, notice down below what we're showing is a plant in a plant cell, and plant cells have a cell wall that surrounds the cell membrane, and the cell wall prevents the expansion of the cell membrane, and so it prevents cell lysis. Plant cells do not have to worry about lysing in hypotonic environments. Instead, plant cells prefer hypotonic environments, and the reason for that is because it leads to high turgor pressure. The cell membrane that is surrounding the cell here is applying pressure to the cell wall that surrounds the cell as well. That allows the cell to take up an upright, healthy structure like what we see here. Plants prefer to be in hypotonic environments, but animal cells do not like hypotonic environments.
Now let's take a look at the second type of environment. If the outside environment that is surrounding a cell is isotonic, then in that scenario, water is going to both enter and exit the cell at equal rates. That means that if water's going in and leaving the cell at equal rates, then the cell is not really going to change in size. This is preferred by animal cells, like our blood cells. Let's take a look at our image down below here in the middle where we're showing you the conditions where the outside environment surrounding the cell is isotonic. In this scenario, water is going to both enters and exit cells at equal rates, which means that the cell is not going to change in size. For animal cells, like these red blood cells that we're showing you here, they prefer isotonic environments. Now taking a look at the plant cell down below when it is in an isotonic environment, water will go into the cell and leave the cell at equal rates, and so the plant cell is not going to change in size. However, notice that the cell membrane here is not applying a lot of pressure to the cell wall. There are these gaps between the cell membrane and the cell wall, and so that means that there's not a really high amount of turgor pressure, and plants don't prefer isotonic environments because it doesn't allow them to have the high turgor pressure that allows them to have their upright healthy formation.
Now let's take a look at the final environment. If the outside environment that is surrounding a cell is hypertonic. In that scenario, water is going to exit cells. When water exits cells, it's almost like deflating a balloon, and so the balloon will get smaller and the cell would also get smaller and shrivel up and shrink down. Also because water is exiting, that's going to cause the cells to dehydrate. They're going to be losing water. This is very similar to how a hyperactive child gets dehydrated. You can think hyper and hyper can lead to dehydration with water leaving the cell. Let's take a look at our image down below over here on the right hand side where the outside environment surrounding the cell is hypertonic. Once again, water is going to be leaving the cell, exiting the cell, And when it does that, the cell itself is going to shrivel up and shrink down and get dehydrated just like all of these cells here. We can say that cells will dehydrate in this environment. This means that animal cells do not prefer hypertonic environments because dehydration can lead to cell death. Now down below here with the plant cell, we're also showing the plant cell in a hypertonic environment. Once again, water is going to be leaving the plant cell in this scenario. In this scenario, what you'll notice is that the cell membrane, which is right here, is not applying a lot of pressure at all to the cell wall that surrounds it. And so there is a really low amount of turgor pressure. That will cause plants to lose their upright healthy structure and begin to wilt and die off as well. Neither animal nor plant cells prefer hypertonic environments because it'll lead to dehydration and low turgor pressure for plants. What you can see here is that plant cells prefer hypotonic environments. Animal cells here prefer isotonic environments, and neither plant nor animal cells prefer hypertonic environments. This concludes our lesson on how environmental tonicity affects cells, and we'll be able to get some practice applying these concepts as we move forward in our course. I'll see you all in our next video.
Plants become turgid when placed in this type of solution:
What would you expect to happen to the cell under the following conditions?
Do you want more practice?
Here’s what students ask on this topic:
What is osmosis and how does it differ from diffusion?
Osmosis is the passive diffusion of water across a semipermeable membrane, requiring no energy. It specifically involves the movement of water from an area of lower solute concentration (hypotonic) to an area of higher solute concentration (hypertonic) to achieve equilibrium. Diffusion, on the other hand, is the movement of solutes from an area of higher concentration to an area of lower concentration, also without energy input. While osmosis focuses on water movement, diffusion pertains to solutes.
 Created using AI
Created using AIHow does tonicity affect the direction of water movement in osmosis?
Tonicity refers to the relative concentration of solutes in solutions. Water moves from hypotonic solutions (lower solute concentration) to hypertonic solutions (higher solute concentration) during osmosis. This movement aims to equalize solute concentrations on both sides of the semipermeable membrane. For example, if the outside of a cell is hypertonic, water will exit the cell, causing it to shrink. Conversely, if the outside is hypotonic, water will enter the cell, causing it to swell.
 Created using AI
Created using AIWhat happens to animal and plant cells in hypotonic, isotonic, and hypertonic environments?
In hypotonic environments, animal cells may lyse (burst) due to excessive water intake, while plant cells benefit from increased turgor pressure, maintaining their structure. In isotonic environments, water enters and exits cells at equal rates, maintaining cell size, which is preferred by animal cells. In hypertonic environments, both animal and plant cells lose water, leading to dehydration and shrinkage. Plant cells lose turgor pressure and may wilt, while animal cells may shrivel.
 Created using AI
Created using AIWhy do plant cells prefer hypotonic environments while animal cells prefer isotonic environments?
Plant cells prefer hypotonic environments because the influx of water increases turgor pressure, which helps maintain their rigid structure and overall health. The cell wall prevents them from bursting. Animal cells, lacking a cell wall, prefer isotonic environments where water movement is balanced, preventing cell lysis or shrinkage. In isotonic conditions, animal cells maintain their normal shape and function optimally.
 Created using AI
Created using AIHow does osmosis contribute to maintaining cell homeostasis?
Osmosis helps maintain cell homeostasis by regulating water balance across cell membranes. By moving water from hypotonic to hypertonic solutions, osmosis ensures that cells do not become too swollen or too shriveled. This balance is crucial for maintaining cell structure, nutrient uptake, and waste removal. Proper osmotic balance allows cells to function efficiently and respond to environmental changes.
 Created using AI
Created using AI