In this video, we're going to begin our lesson on functional groups. Now, functional groups, as their name implies, are really just groups of atoms that are reactive or functional, and they're also commonly found together within biomolecules. And so, moving forward in our course, when we're talking about different classes of biomolecules, we're going to see lots and lots of functional groups appear, and so it's important to get to know these functional groups pretty well. Now, the functional groups are typically going to extend off of the carbon backbone of a molecule, and so notice down below in our image we're going to represent the carbon backbones of the molecules by using these squiggly lines throughout which again represent the carbon backbone of the rest of the molecule, and so the functional group is always going to be extending off of some type of carbon backbone. Now, throughout all of biology, there are a lot of different types of functional groups. However, in a typical biology course like yours, you're likely only going to need to know 7 functional groups that are pretty common in biology. And so notice down below in our table, we have these 7 functional groups for you all, to know. And so the very first functional group that you all should know is the methyl group. And the methyl group is just when you have a carbon atom branching off of a carbon backbone, and this carbon atom is covalently attached to 3 other hydrogen atoms. And so that's it for the methyl group over here. And typically these methyl groups, what we'll see is that they're going to be found in lipids and things like that. So we'll be able to see those a little bit later in our course. Now, the second functional group that you all should know is the hydroxyl group. And the hydroxyl group, as you can kind of see with its name, it has the "oxy" in here for an oxygen atom and it has the "hydro" in here for the hydrogen atom, and that's exactly what it is. The hydroxyl group is going to have an oxygen atom branching off of a chain that's also bound to a hydrogen atom. And so an OH group like what you see here is going to represent the hydroxyl group, and we'll see hydroxyl groups in lots of different types of molecules, including carbohydrates. Now, over here what we have is the 3rd class of the functional group or the 3rd functional group, and that is the carbonyl group. And the carbonyl group is whenever you have a carbon atom that is double bonded, has a double bond to an oxygen atom. And so whenever you have a carbon atom double bonded to an oxygen atom that is a carbonyl group. And so once again these are fairly common throughout different types of biomolecules. Now, over here the 4th functional group that we have here is going to be the carboxyl group. Now I admit, at first glance, the carbonyl and the carboxyl sound very, very similar. However, the carboxyl, what can help you remember is that boxes like this box over here are used to store things and really the carboxyl is a combination of the hydroxyl group and the carbonyl group as well. And so, it's going to be, a combination of both. So, notice that the carbonyl group is present here in the carboxyl because there's a carbon double bonded to an oxygen just like what we saw over here. But in addition to the carbonyl group, there's also a hydroxyl group over here within this carboxyl group and so there's an OH over here as well. And so when you see a carbon double bonded to an oxygen and then that same carbon is bonded to a hydroxyl group, together we refer to this entire thing as a carboxyl group. And so, the 5th functional group that we have down below here is going to be the amino group. And so the amino group, you can think has this "n" in it, and really that's representing the nitrogen atom that is found within the amino groups. And so this is an example of an amino group. Now the 6th functional group that we have here is going to be the phosphate group, and the phosphate group looks pretty complex here. However, it's pretty easily identifiable because it's the only one of the 7 that has a phosphorus atom like what we see here. And so, this is it for the phosphate group. And once again, we'll see these functional groups throughout many different types of biomolecules moving forward in our course. And then the 7th and final functional group that you all should be aware of is the sulfhydryl group. And so the sulfhydryl group, as its name implies, with the "sulf" here, it's going to have a sulfur atom. And the "hydra" prefix here is going to have a hydrogen atom. And that's exactly what the sulfhydryl group is, a sulfur and a hydrogen atom just like what we see here. And so these are the 7 functional groups that would be good to commit to memory because moving forward in our course, we're going to be able to refer to all of these different functional groups. Now some of them you might need to commit to memory in terms of the structures, but others you might need to commit to memory just in terms of being able to identify or recognize them. For example, the phosphate group might be one that you would just need to identify and recognize, but you'll have to ask your professor to figure out exactly which functional groups they want you to be aware of. Now, this here concludes our introduction to functional groups and we'll be able to get some practice applying the concepts that we've learned in our next few videos. So I'll see you all there.
- 1. Introduction to Microbiology3h 21m
- Introduction to Microbiology16m
- Introduction to Taxonomy26m
- Scientific Naming of Organisms9m
- Members of the Bacterial World10m
- Introduction to Bacteria9m
- Introduction to Archaea10m
- Introduction to Eukarya20m
- Acellular Infectious Agents: Viruses, Viroids & Prions19m
- Importance of Microorganisms20m
- Scientific Method27m
- Experimental Design30m
- 2. Disproving Spontaneous Generation1h 18m
- 3. Chemical Principles of Microbiology3h 38m
- 4. Water1h 28m
- 5. Molecules of Microbiology2h 23m
- 6. Cell Membrane & Transport3h 28m
- Cell Envelope & Biological Membranes12m
- Bacterial & Eukaryotic Cell Membranes8m
- Archaeal Cell Membranes18m
- Types of Membrane Proteins8m
- Concentration Gradients and Diffusion9m
- Introduction to Membrane Transport14m
- Passive vs. Active Transport13m
- Osmosis33m
- Simple and Facilitated Diffusion17m
- Active Transport30m
- ABC Transporters11m
- Group Translocation7m
- Types of Small Molecule Transport Review9m
- Endocytosis and Exocytosis15m
- 7. Prokaryotic Cell Structures & Functions5h 52m
- Prokaryotic & Eukaryotic Cells26m
- Binary Fission11m
- Generation Times16m
- Bacterial Cell Morphology & Arrangements35m
- Overview of Prokaryotic Cell Structure10m
- Introduction to Bacterial Cell Walls26m
- Gram-Positive Cell Walls11m
- Gram-Negative Cell Walls20m
- Gram-Positive vs. Gram-Negative Cell Walls11m
- The Glycocalyx: Capsules & Slime Layers12m
- Introduction to Biofilms6m
- Pili18m
- Fimbriae & Hami7m
- Introduction to Prokaryotic Flagella12m
- Prokaryotic Flagellar Structure18m
- Prokaryotic Flagellar Movement11m
- Proton Motive Force Drives Flagellar Motility5m
- Chemotaxis14m
- Review of Prokaryotic Surface Structures8m
- Prokaryotic Ribosomes16m
- Introduction to Bacterial Plasmids13m
- Cell Inclusions9m
- Endospores16m
- Sporulation5m
- Germination5m
- 8. Eukaryotic Cell Structures & Functions2h 18m
- 9. Microscopes2h 46m
- Introduction to Microscopes8m
- Magnification, Resolution, & Contrast10m
- Introduction to Light Microscopy5m
- Light Microscopy: Bright-Field Microscopes23m
- Light Microscopes that Increase Contrast16m
- Light Microscopes that Detect Fluorescence16m
- Electron Microscopes14m
- Reviewing the Different Types of Microscopes10m
- Introduction to Staining5m
- Simple Staining14m
- Differential Staining6m
- Other Types of Staining11m
- Reviewing the Types of Staining8m
- Gram Stain13m
- 10. Dynamics of Microbial Growth4h 36m
- Biofilms16m
- Growing a Pure Culture5m
- Microbial Growth Curves in a Closed System21m
- Temperature Requirements for Microbial Growth18m
- Oxygen Requirements for Microbial Growth22m
- pH Requirements for Microbial Growth8m
- Osmolarity Factors for Microbial Growth14m
- Reviewing the Environmental Factors of Microbial Growth12m
- Nutritional Factors of Microbial Growth30m
- Growth Factors4m
- Introduction to Cultivating Microbial Growth5m
- Types of Solid Culture Media4m
- Plating Methods16m
- Measuring Growth by Direct Cell Counts9m
- Measuring Growth by Plate Counts14m
- Measuring Growth by Membrane Filtration6m
- Measuring Growth by Biomass15m
- Introduction to the Types of Culture Media5m
- Chemically Defined Media3m
- Complex Media4m
- Selective Media5m
- Differential Media9m
- Reducing Media4m
- Enrichment Media7m
- Reviewing the Types of Culture Media8m
- 11. Controlling Microbial Growth4h 10m
- Introduction to Controlling Microbial Growth29m
- Selecting a Method to Control Microbial Growth44m
- Physical Methods to Control Microbial Growth49m
- Review of Physical Methods to Control Microbial Growth7m
- Chemical Methods to Control Microbial Growth16m
- Chemicals Used to Control Microbial Growth6m
- Liquid Chemicals: Alcohols, Aldehydes, & Biguanides15m
- Liquid Chemicals: Halogens12m
- Liquid Chemicals: Surface-Active Agents17m
- Other Types of Liquid Chemicals14m
- Chemical Gases: Ethylene Oxide, Ozone, & Formaldehyde13m
- Review of Chemicals Used to Control Microbial Growth11m
- Chemical Preservation of Perishable Products10m
- 12. Microbial Metabolism5h 16m
- Introduction to Energy15m
- Laws of Thermodynamics15m
- Chemical Reactions9m
- ATP20m
- Enzymes14m
- Enzyme Activation Energy9m
- Enzyme Binding Factors9m
- Enzyme Inhibition10m
- Introduction to Metabolism8m
- Negative & Positive Feedback7m
- Redox Reactions22m
- Introduction to Aerobic Cellular Respiration25m
- Types of Phosphorylation12m
- Glycolysis19m
- Entner-Doudoroff Pathway11m
- Pentose-Phosphate Pathway10m
- Pyruvate Oxidation8m
- Krebs Cycle16m
- Electron Transport Chain19m
- Chemiosmosis7m
- Review of Aerobic Cellular Respiration19m
- Fermentation & Anaerobic Respiration23m
- 13. Photosynthesis2h 31m
- 14. DNA Replication2h 25m
- 15. Central Dogma & Gene Regulation7h 14m
- Central Dogma7m
- Introduction to Transcription20m
- Steps of Transcription22m
- Transcription Termination in Prokaryotes7m
- Eukaryotic RNA Processing and Splicing20m
- Introduction to Types of RNA9m
- Genetic Code25m
- Introduction to Translation30m
- Steps of Translation23m
- Review of Transcription vs. Translation12m
- Prokaryotic Gene Expression21m
- Review of Prokaryotic vs. Eukaryotic Gene Expression13m
- Introduction to Regulation of Gene Expression13m
- Prokaryotic Gene Regulation via Operons27m
- The Lac Operon21m
- Glucose's Impact on Lac Operon25m
- The Trp Operon20m
- Review of the Lac Operon & Trp Operon11m
- Introduction to Eukaryotic Gene Regulation9m
- Eukaryotic Chromatin Modifications16m
- Eukaryotic Transcriptional Control22m
- Eukaryotic Post-Transcriptional Regulation28m
- Post-Translational Modification6m
- Eukaryotic Post-Translational Regulation13m
- 16. Microbial Genetics4h 44m
- Introduction to Microbial Genetics11m
- Introduction to Mutations20m
- Methods of Inducing Mutations15m
- Prototrophs vs. Auxotrophs13m
- Mutant Detection25m
- The Ames Test14m
- Introduction to DNA Repair5m
- DNA Repair Mechanisms37m
- Horizontal Gene Transfer18m
- Bacterial Transformation11m
- Transduction32m
- Introduction to Conjugation6m
- Conjugation: F Plasmids18m
- Conjugation: Hfr & F' Cells19m
- Genome Variability21m
- CRISPR CAS11m
- 17. Biotechnology3h 0m
- 18. Viruses, Viroids, & Prions4h 56m
- Introduction to Viruses20m
- Introduction to Bacteriophage Infections14m
- Bacteriophage: Lytic Phage Infections12m
- Bacteriophage: Lysogenic Phage Infections17m
- Bacteriophage: Filamentous Phage Infections8m
- Plaque Assays9m
- Introduction to Animal Virus Infections10m
- Animal Viruses: 1. Attachment to the Host Cell7m
- Animal Viruses: 2. Entry & Uncoating in the Host Cell19m
- Animal Viruses: 3. Synthesis & Replication22m
- Animal Viruses: DNA Virus Synthesis & Replication14m
- Animal Viruses: RNA Virus Synthesis & Replication22m
- Animal Viruses: Antigenic Drift vs. Antigenic Shift9m
- Animal Viruses: Reverse-Transcribing Virus Synthesis & Replication9m
- Animal Viruses: 4. Assembly Inside Host Cell8m
- Animal Viruses: 5. Release from Host Cell15m
- Acute vs. Persistent Viral Infections25m
- COVID-19 (SARS-CoV-2)14m
- Plant Viruses12m
- Viroids6m
- Prions13m
- 19. Innate Immunity7h 15m
- Introduction to Immunity8m
- Introduction to Innate Immunity17m
- Introduction to First-Line Defenses5m
- Physical Barriers in First-Line Defenses: Skin13m
- Physical Barriers in First-Line Defenses: Mucous Membrane9m
- First-Line Defenses: Chemical Barriers24m
- First-Line Defenses: Normal Microflora5m
- Introduction to Cells of the Immune System15m
- Cells of the Immune System: Granulocytes29m
- Cells of the Immune System: Agranulocytes25m
- Introduction to Cell Communication5m
- Cell Communication: Surface Receptors & Adhesion Molecules16m
- Cell Communication: Cytokines27m
- Pattern Recognition Receptors (PRRs)45m
- Introduction to the Complement System24m
- Activation Pathways of the Complement System23m
- Effects of the Complement System23m
- Review of the Complement System12m
- Phagoctytosis21m
- Introduction to Inflammation18m
- Steps of the Inflammatory Response26m
- Fever8m
- Interferon Response25m
- 20. Adaptive Immunity7h 14m
- Introduction to Adaptive Immunity32m
- Antigens12m
- Introduction to T Lymphocytes38m
- Major Histocompatibility Complex Molecules20m
- Activation of T Lymphocytes21m
- Functions of T Lymphocytes25m
- Review of Cytotoxic vs Helper T Cells13m
- Introduction to B Lymphocytes27m
- Antibodies14m
- Classes of Antibodies35m
- Outcomes of Antibody Binding to Antigen15m
- T Dependent & T Independent Antigens21m
- Clonal Selection20m
- Antibody Class Switching17m
- Affinity Maturation14m
- Primary and Secondary Response of Adaptive Immunity21m
- Immune Tolerance28m
- Regulatory T Cells10m
- Natural Killer Cells16m
- Review of Adaptive Immunity25m
- 21. Principles of Disease6h 57m
- Symbiotic Relationships12m
- The Human Microbiome46m
- Characteristics of Infectious Disease47m
- Stages of Infectious Disease Progression26m
- Koch's Postulates26m
- Molecular Koch's Postulates11m
- Bacterial Pathogenesis36m
- Introduction to Pathogenic Toxins6m
- Exotoxins Cause Damage to the Host40m
- Endotoxin Causes Damage to the Host13m
- Exotoxins vs. Endotoxin Review13m
- Immune Response Damage to the Host15m
- Introduction to Avoiding Host Defense Mechanisms8m
- 1) Hide Within Host Cells5m
- 2) Avoiding Phagocytosis31m
- 3) Surviving Inside Phagocytic Cells10m
- 4) Avoiding Complement System9m
- 5) Avoiding Antibodies25m
- Viruses Evade the Immune Response27m
Functional Groups - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIFunctional groups are specific groups of atoms that confer reactivity to biomolecules, typically extending from a carbon backbone. Key functional groups include the methyl group (–CH3), hydroxyl group (–OH), carbonyl group (C=O), carboxyl group (–COOH), amino group (–NH2), phosphate group (–PO4), and sulfhydryl group (–SH). Understanding these groups is essential for recognizing their roles in various biomolecules, such as lipids and carbohydrates, and their significance in biological processes.
Functional Groups
Video transcript
Functional Groups Example 1
Video transcript
Alright. So here we have an example problem that's asking which functional group listed down below over here is not present in this molecule over here. And so notice that the very first functional group listed is carboxyl, and recall that the carboxyl has the box in it which we know is going to store 2 other functional groups, the carbonyl group and the hydroxyl group. And so notice over here we have a carbon double bonded to an oxygen, and we also have a hydroxyl group branching off. And so together when we see this format like this, we refer to it as a carboxyl group. So the carboxyl group is present here and we can go ahead and cross it off and label it, as we see right here. Let's label this as carboxyl.
Then moving on, what you'll also notice is that we have an OH group extending off of a carbon. And so the OH, recall, is going to be the hydroxyl group because it has the oxy in it for the oxygen and the hydro for the hydrogen. And so, this here is going to be a hydroxyl group. And so because a hydroxyl group is here, we can go ahead and cross off option c as well.
And then recall that amino groups are going to have a nitrogen atom in them, and so notice that the nitrogen atom is right here and this here represents the amino group. And so the only one that is not present here is going to be the sulfhydryl group, which recall has a sulfur atom and a hydrogen atom. But there are no sulfur atoms at all throughout here, so this is going to be the one that is not present in the molecule. So we can go ahead and indicate that b here is the correct answer for this example and that concludes this example, so I'll see you all in our next video.
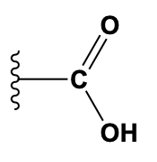
What is the name of the functional group shown in the figure?
a) Carbonyl.
b) Ketone.
c) Carboxyl.
d) Methyl.
e) Phosphate.
All of the following are examples of functional groups in biology except:
a) -CH3.
b) -COOH.
c) -H2O.
d) -NH2.
e) -OH.
Do you want more practice?
Here’s what students ask on this topic:
What are functional groups in organic chemistry?
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. They typically extend from a carbon backbone and confer specific reactivity and properties to the molecule. Common functional groups include the methyl group (–CH3), hydroxyl group (–OH), carbonyl group (C=O), carboxyl group (–COOH), amino group (–NH2), phosphate group (–PO4), and sulfhydryl group (–SH). Understanding these groups is essential for recognizing their roles in various biomolecules, such as lipids and carbohydrates, and their significance in biological processes.
 Created using AI
Created using AIWhy are functional groups important in biology?
Functional groups are crucial in biology because they determine the properties and reactivity of biomolecules. They play a key role in the structure and function of molecules like proteins, nucleic acids, carbohydrates, and lipids. For example, the hydroxyl group (–OH) is important in carbohydrates, while the amino group (–NH2) is essential in amino acids, the building blocks of proteins. Functional groups also participate in chemical reactions that are vital for life, such as enzyme catalysis, energy transfer, and cell signaling.
 Created using AI
Created using AIWhat is the difference between a carbonyl group and a carboxyl group?
The carbonyl group (C=O) consists of a carbon atom double-bonded to an oxygen atom. It is found in aldehydes and ketones. The carboxyl group (–COOH) is a combination of a carbonyl group and a hydroxyl group (–OH) attached to the same carbon atom. It is found in carboxylic acids and is important in amino acids and fatty acids. The carboxyl group can donate a proton (H+), making it acidic, whereas the carbonyl group does not have this property.
 Created using AI
Created using AIHow do you identify a phosphate group in a molecule?
A phosphate group (–PO4) is identifiable by the presence of a phosphorus atom bonded to four oxygen atoms. It is unique among common functional groups because it contains phosphorus. Phosphate groups are crucial in energy transfer molecules like ATP (adenosine triphosphate) and in the backbone of nucleic acids like DNA and RNA. They often participate in energy transfer and signaling processes within cells.
 Created using AI
Created using AIWhat role does the amino group play in proteins?
The amino group (–NH2) is a fundamental component of amino acids, which are the building blocks of proteins. In amino acids, the amino group is attached to the alpha carbon along with a carboxyl group (–COOH), a hydrogen atom, and a variable R group (side chain). The amino group can act as a base, accepting a proton (H+), which is important for the protein's structure and function. It also participates in peptide bond formation during protein synthesis.
 Created using AI
Created using AI