In this video, we're going to begin our lesson on dideoxy sequencing. Dideoxy sequencing is a specific DNA sequencing method or technique that uses ddNTPs or dideoxynucleotides as elongation terminators to help determine the sequence of the DNA. Dideoxy sequencing was actually first discovered way back in 1977 by a scientist named Frederick Sanger, and for that reason, it is sometimes commonly referred to as Sanger Sequencing. This was the first method of DNA sequencing that uses these dideoxynucleotides. We'll be able to talk more about dideoxy sequencing and Sanger sequencing as we continue to move forward in our course. So, I'll see you all in our next video.
- 1. Introduction to Microbiology3h 21m
- Introduction to Microbiology16m
- Introduction to Taxonomy26m
- Scientific Naming of Organisms9m
- Members of the Bacterial World10m
- Introduction to Bacteria9m
- Introduction to Archaea10m
- Introduction to Eukarya20m
- Acellular Infectious Agents: Viruses, Viroids & Prions19m
- Importance of Microorganisms20m
- Scientific Method27m
- Experimental Design30m
- 2. Disproving Spontaneous Generation1h 18m
- 3. Chemical Principles of Microbiology3h 38m
- 4. Water1h 28m
- 5. Molecules of Microbiology2h 23m
- 6. Cell Membrane & Transport3h 28m
- Cell Envelope & Biological Membranes12m
- Bacterial & Eukaryotic Cell Membranes8m
- Archaeal Cell Membranes18m
- Types of Membrane Proteins8m
- Concentration Gradients and Diffusion9m
- Introduction to Membrane Transport14m
- Passive vs. Active Transport13m
- Osmosis33m
- Simple and Facilitated Diffusion17m
- Active Transport30m
- ABC Transporters11m
- Group Translocation7m
- Types of Small Molecule Transport Review9m
- Endocytosis and Exocytosis15m
- 7. Prokaryotic Cell Structures & Functions5h 52m
- Prokaryotic & Eukaryotic Cells26m
- Binary Fission11m
- Generation Times16m
- Bacterial Cell Morphology & Arrangements35m
- Overview of Prokaryotic Cell Structure10m
- Introduction to Bacterial Cell Walls26m
- Gram-Positive Cell Walls11m
- Gram-Negative Cell Walls20m
- Gram-Positive vs. Gram-Negative Cell Walls11m
- The Glycocalyx: Capsules & Slime Layers12m
- Introduction to Biofilms6m
- Pili18m
- Fimbriae & Hami7m
- Introduction to Prokaryotic Flagella12m
- Prokaryotic Flagellar Structure18m
- Prokaryotic Flagellar Movement11m
- Proton Motive Force Drives Flagellar Motility5m
- Chemotaxis14m
- Review of Prokaryotic Surface Structures8m
- Prokaryotic Ribosomes16m
- Introduction to Bacterial Plasmids13m
- Cell Inclusions9m
- Endospores16m
- Sporulation5m
- Germination5m
- 8. Eukaryotic Cell Structures & Functions2h 18m
- 9. Microscopes2h 46m
- Introduction to Microscopes8m
- Magnification, Resolution, & Contrast10m
- Introduction to Light Microscopy5m
- Light Microscopy: Bright-Field Microscopes23m
- Light Microscopes that Increase Contrast16m
- Light Microscopes that Detect Fluorescence16m
- Electron Microscopes14m
- Reviewing the Different Types of Microscopes10m
- Introduction to Staining5m
- Simple Staining14m
- Differential Staining6m
- Other Types of Staining11m
- Reviewing the Types of Staining8m
- Gram Stain13m
- 10. Dynamics of Microbial Growth4h 36m
- Biofilms16m
- Growing a Pure Culture5m
- Microbial Growth Curves in a Closed System21m
- Temperature Requirements for Microbial Growth18m
- Oxygen Requirements for Microbial Growth22m
- pH Requirements for Microbial Growth8m
- Osmolarity Factors for Microbial Growth14m
- Reviewing the Environmental Factors of Microbial Growth12m
- Nutritional Factors of Microbial Growth30m
- Growth Factors4m
- Introduction to Cultivating Microbial Growth5m
- Types of Solid Culture Media4m
- Plating Methods16m
- Measuring Growth by Direct Cell Counts9m
- Measuring Growth by Plate Counts14m
- Measuring Growth by Membrane Filtration6m
- Measuring Growth by Biomass15m
- Introduction to the Types of Culture Media5m
- Chemically Defined Media3m
- Complex Media4m
- Selective Media5m
- Differential Media9m
- Reducing Media4m
- Enrichment Media7m
- Reviewing the Types of Culture Media8m
- 11. Controlling Microbial Growth4h 10m
- Introduction to Controlling Microbial Growth29m
- Selecting a Method to Control Microbial Growth44m
- Physical Methods to Control Microbial Growth49m
- Review of Physical Methods to Control Microbial Growth7m
- Chemical Methods to Control Microbial Growth16m
- Chemicals Used to Control Microbial Growth6m
- Liquid Chemicals: Alcohols, Aldehydes, & Biguanides15m
- Liquid Chemicals: Halogens12m
- Liquid Chemicals: Surface-Active Agents17m
- Other Types of Liquid Chemicals14m
- Chemical Gases: Ethylene Oxide, Ozone, & Formaldehyde13m
- Review of Chemicals Used to Control Microbial Growth11m
- Chemical Preservation of Perishable Products10m
- 12. Microbial Metabolism5h 16m
- Introduction to Energy15m
- Laws of Thermodynamics15m
- Chemical Reactions9m
- ATP20m
- Enzymes14m
- Enzyme Activation Energy9m
- Enzyme Binding Factors9m
- Enzyme Inhibition10m
- Introduction to Metabolism8m
- Negative & Positive Feedback7m
- Redox Reactions22m
- Introduction to Aerobic Cellular Respiration25m
- Types of Phosphorylation12m
- Glycolysis19m
- Entner-Doudoroff Pathway11m
- Pentose-Phosphate Pathway10m
- Pyruvate Oxidation8m
- Krebs Cycle16m
- Electron Transport Chain19m
- Chemiosmosis7m
- Review of Aerobic Cellular Respiration19m
- Fermentation & Anaerobic Respiration23m
- 13. Photosynthesis2h 31m
- 14. DNA Replication2h 25m
- 15. Central Dogma & Gene Regulation7h 14m
- Central Dogma7m
- Introduction to Transcription20m
- Steps of Transcription22m
- Transcription Termination in Prokaryotes7m
- Eukaryotic RNA Processing and Splicing20m
- Introduction to Types of RNA9m
- Genetic Code25m
- Introduction to Translation30m
- Steps of Translation23m
- Review of Transcription vs. Translation12m
- Prokaryotic Gene Expression21m
- Review of Prokaryotic vs. Eukaryotic Gene Expression13m
- Introduction to Regulation of Gene Expression13m
- Prokaryotic Gene Regulation via Operons27m
- The Lac Operon21m
- Glucose's Impact on Lac Operon25m
- The Trp Operon20m
- Review of the Lac Operon & Trp Operon11m
- Introduction to Eukaryotic Gene Regulation9m
- Eukaryotic Chromatin Modifications16m
- Eukaryotic Transcriptional Control22m
- Eukaryotic Post-Transcriptional Regulation28m
- Post-Translational Modification6m
- Eukaryotic Post-Translational Regulation13m
- 16. Microbial Genetics4h 44m
- Introduction to Microbial Genetics11m
- Introduction to Mutations20m
- Methods of Inducing Mutations15m
- Prototrophs vs. Auxotrophs13m
- Mutant Detection25m
- The Ames Test14m
- Introduction to DNA Repair5m
- DNA Repair Mechanisms37m
- Horizontal Gene Transfer18m
- Bacterial Transformation11m
- Transduction32m
- Introduction to Conjugation6m
- Conjugation: F Plasmids18m
- Conjugation: Hfr & F' Cells19m
- Genome Variability21m
- CRISPR CAS11m
- 17. Biotechnology3h 0m
- 18. Viruses, Viroids, & Prions4h 56m
- Introduction to Viruses20m
- Introduction to Bacteriophage Infections14m
- Bacteriophage: Lytic Phage Infections12m
- Bacteriophage: Lysogenic Phage Infections17m
- Bacteriophage: Filamentous Phage Infections8m
- Plaque Assays9m
- Introduction to Animal Virus Infections10m
- Animal Viruses: 1. Attachment to the Host Cell7m
- Animal Viruses: 2. Entry & Uncoating in the Host Cell19m
- Animal Viruses: 3. Synthesis & Replication22m
- Animal Viruses: DNA Virus Synthesis & Replication14m
- Animal Viruses: RNA Virus Synthesis & Replication22m
- Animal Viruses: Antigenic Drift vs. Antigenic Shift9m
- Animal Viruses: Reverse-Transcribing Virus Synthesis & Replication9m
- Animal Viruses: 4. Assembly Inside Host Cell8m
- Animal Viruses: 5. Release from Host Cell15m
- Acute vs. Persistent Viral Infections25m
- COVID-19 (SARS-CoV-2)14m
- Plant Viruses12m
- Viroids6m
- Prions13m
- 19. Innate Immunity7h 15m
- Introduction to Immunity8m
- Introduction to Innate Immunity17m
- Introduction to First-Line Defenses5m
- Physical Barriers in First-Line Defenses: Skin13m
- Physical Barriers in First-Line Defenses: Mucous Membrane9m
- First-Line Defenses: Chemical Barriers24m
- First-Line Defenses: Normal Microflora5m
- Introduction to Cells of the Immune System15m
- Cells of the Immune System: Granulocytes29m
- Cells of the Immune System: Agranulocytes25m
- Introduction to Cell Communication5m
- Cell Communication: Surface Receptors & Adhesion Molecules16m
- Cell Communication: Cytokines27m
- Pattern Recognition Receptors (PRRs)45m
- Introduction to the Complement System24m
- Activation Pathways of the Complement System23m
- Effects of the Complement System23m
- Review of the Complement System12m
- Phagoctytosis21m
- Introduction to Inflammation18m
- Steps of the Inflammatory Response26m
- Fever8m
- Interferon Response25m
- 20. Adaptive Immunity7h 14m
- Introduction to Adaptive Immunity32m
- Antigens12m
- Introduction to T Lymphocytes38m
- Major Histocompatibility Complex Molecules20m
- Activation of T Lymphocytes21m
- Functions of T Lymphocytes25m
- Review of Cytotoxic vs Helper T Cells13m
- Introduction to B Lymphocytes27m
- Antibodies14m
- Classes of Antibodies35m
- Outcomes of Antibody Binding to Antigen15m
- T Dependent & T Independent Antigens21m
- Clonal Selection20m
- Antibody Class Switching17m
- Affinity Maturation14m
- Primary and Secondary Response of Adaptive Immunity21m
- Immune Tolerance28m
- Regulatory T Cells10m
- Natural Killer Cells16m
- Review of Adaptive Immunity25m
- 21. Principles of Disease6h 57m
- Symbiotic Relationships12m
- The Human Microbiome46m
- Characteristics of Infectious Disease47m
- Stages of Infectious Disease Progression26m
- Koch's Postulates26m
- Molecular Koch's Postulates11m
- Bacterial Pathogenesis36m
- Introduction to Pathogenic Toxins6m
- Exotoxins Cause Damage to the Host40m
- Endotoxin Causes Damage to the Host13m
- Exotoxins vs. Endotoxin Review13m
- Immune Response Damage to the Host15m
- Introduction to Avoiding Host Defense Mechanisms8m
- 1) Hide Within Host Cells5m
- 2) Avoiding Phagocytosis31m
- 3) Surviving Inside Phagocytic Cells10m
- 4) Avoiding Complement System9m
- 5) Avoiding Antibodies25m
- Viruses Evade the Immune Response27m
Dideoxy Sequencing - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIDideoxy sequencing, also known as Sanger sequencing, is a DNA sequencing method that utilizes dideoxynucleotides (ddNTPs) as chain terminators. Key components include template DNA, DNA polymerase, DNA primers, deoxyribonucleotides, and ddNTPs. The process involves chain termination PCR, producing DNA fragments that are separated by size using gel electrophoresis. The resulting gel can be analyzed to determine the DNA sequence, revealing the complementary sequence of the unknown template. This method is foundational in molecular biology for understanding genetic information.
Dideoxy Sequencing
Video transcript
Components of Dideoxy Sequencing
Video transcript
This video, we're going to focus on the specific components that are needed for dideoxy sequencing, but we're not going to get into the actual process of dideoxy sequencing until we get to another video later on in our course. In this video, we're only focusing on the specific components. And so the components that are needed in dideoxy sequencing reactions include the following 5 components that we have labeled down below a through e. And of course, these labels a through e that you see here correspond with the labels a through e that you see down below in our image. And so the very first component that is needed in a specific dideoxy sequencing reaction is, of course, going to be the unknown template DNA of interest whose sequence we do not know, which is why we want to conduct dideoxy sequencing. And so over here, what we have is our template DNA, and you can see this is the DNA here. And we do not know the sequence of the DNA, which is why we want to conduct this sequencing to figure out what is the sequence of this DNA. So, that is the first component.
Then, the second component that we are going to need is actually a DNA polymerase, which you might recall is an enzyme that polymerizes or builds DNA. And so DNA polymerase is the main enzyme that's needed for DNA replication. And so, what you'll see here down below is the second component that we need is the DNA polymerase. And of course, this is the components of dideoxy sequencing here. Okay?
Now, the third component that is needed is going to be DNA primers. And these DNA primers are going to anneal to the template strand. And so recall that DNA primers were used in the specific technique that we talked about in our previous lesson videos called polymerase chain reaction or PCR. And so, what we'll see moving forward is that dideoxy sequencing is actually going to use a special type of PCR, and we'll see that moving forward. Now the DNA primers you can see down below right here, and there will need to be, of course, 2 DNA primers.
Now, the 4th component that is going to be needed are all 4 DNA deoxyribonucleotides. And so these are going to be the normal deoxyribonucleotides that are used in DNA, during normal cellular DNA replication. And so, these deoxyribonucleotides include dATP, dTTP, dGTP, and dCTP. So, this is basically the adenine, thymine, guanine, and cytosine. And then, you can see these deoxyribonucleotides down below here. And again, these are the normal DNA nucleotides.
And then, of course, for dideoxy sequencing where the 5th and final component that we're going to need is a small amount of a single dideoxyribonucleotide. And so, this is the special type of nucleotide. The ddATPs, ddTTPs, ddGTPs, and ddCTPs. And so in a particular dideoxy sequencing reaction within a single test tube, we would only want to use just one single nucleotide. And so we would have to separate these reactions using different test tubes, to use different dideoxynucleotides. And we'll talk more about this as we move forward in our course.
And so recall that these ddNTPs, these dideoxyribonucleotides, they are going to terminate the DNA synthesis due to the presence of a 3 prime hydrogen atom or group. And so, what you'll notice is down below right here, you can see that these are the dideoxyribo DNA nucleotides. And so these are the special chain terminating ddNTPs, dideoxynucleotides. And so these are really the 5 major components that are needed for dideoxy sequencing and these are the components that you will see mentioned as we move forward and talk more about the specifics of the process of dideoxy sequencing. And so for now, this here concludes this lesson and, we'll be able to talk more about this as we move forward.
Which of the following is NOT required for the reactions in dideoxy sequencing?
Chain-Termination PCR
Video transcript
In this video, we're going to continue to talk about dideoxy sequencing as we talk about the chain termination PCR steps or the chain termination polymerase chain reaction steps. Recall from our previous lesson videos that we already talked about polymerase chain reaction or PCR. Be sure to check out those older videos on PCR, polymerase chain reaction before you continue here. Also, recall from our previous lesson videos that DNA synthesis reaction is actually terminated when a dideoxynucleotide or a ddNTP is added to the 3' end of the growing DNA strand. The use of these ddNTPs to terminate the chain is really what chain termination PCR relies on.
In the first two steps of dideoxysequencing, it requires setting up a chain termination PCR which is really just a PCR reaction that's going to include small amounts of ddNTPs. In this first step, we're going to need to set up 4 separate reactions in 4 separate test tubes. Notice down below in our image on the left-hand side, you can see that we've got these 4 different test tubes where we're going to set up 4 different reactions. Each of these 4 separate reactions that are being set up, they're each going to contain all of the components that are needed for a normal PCR and they're also going to contain a small amount of a different ddNTP. This small amount of different ddNTP is what distinguishes one tube from another test tube.
In one test tube, it has all of the components for a normal PCR, but it also includes the ddNTP for cytosine. This is going to provide chain termination at all of the cytosine nucleotides upon amplification of the DNA. In another test tube, it also has all of the normal components of our PCR, but it differs from the previous one in that it has the ddNTP for thymine. Chain termination in this tube is going to occur at all of the thymine nucleotides. Another test tube is going to contain all of the components for a normal PCR, but it's also going to contain a small amount of the ddNTP for adenine. Similarly, the last test tube will have all of the components for a normal PCR, but it differs in that it has a small amount of the ddNTPs for guanine. These four test tubes differ from each other in the small amount of the different ddNTP that's being added. The ddNTP is going to lead to chain termination at that specific nucleotide: C's in the first, T's in the second, A's in the third, and G's in the fourth.
At the top here is mystery DNA. The mystery DNA is the specific DNA sequence that we want to be able to sequence and determine the sequence of this mysterious DNA whose sequence we do not know at the moment. Dideoxy sequencing can help us determine the sequence of this mystery DNA, and we have to set up chain termination PCR. This mystery DNA is going to serve as the template DNA for amplification during this PCR. In step number 2, what we have is conducting the actual PCR reaction, the chain termination PCR reaction. DNA synthesis is actually going to produce a bunch of fragments of DNA. The reason that it produces fragments of DNA is because of the ddNTPs which is going to terminate the DNA synthesis and create a bunch of fragments that terminate at the specific nucleotides that are indicated in each tube.
Upon conducting the actual chain termination PCR, the DNA is going to be replicated. What you'll notice is that there are a bunch of different-sized fragments of DNA that are going to be generated. For each of these fragments, you'll notice that in the background there's a different-colored background at the end of each of these chains. The one that has that different-colored background here would represent the ddNTP that's being incorporated and terminating the chain at that nucleotide. When these PCR products are analyzed, it can actually reveal the sequence of the mystery DNA. This concludes our introduction to the chain termination PCR, and we'll be able to get some practice applying this and then talk about exactly how these PCR products can be analyzed to reveal the sequence of the DNA in our next video. I'll see you all there.
Determining the DNA Sequence from a Gel
Video transcript
So after chain termination PCR, the next steps in dideoxy sequencing involve determining the DNA sequence from a gel. And so in the final two steps of didactic sequencing, the DNA sequence is finally going to be determined. And so in step number 3, which is a continuation of the chain termination PCR step from our previous lesson video, the fragments from all 4 chain termination PCR reactions are going to be separated by size by using gel electrophoresis. And so if we take a look at our image down below, notice on the far left hand side what we have are the products of our chain termination PCR. And these products are going to be different-sized fragments. And so these different-sized PCR products or different-sized fragments, they can be separated via gel electrophoresis. And recall from our previous lesson videos that gel electrophoresis is going to load each of the different samples towards the top of the gel in specific wells, and then it will be separating the fragments within each lane based on their size.
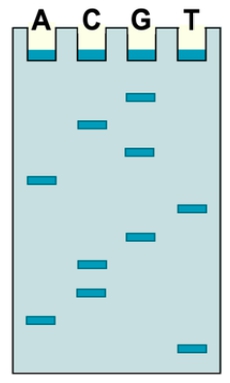
And so in step number 4, what we need to do is determine the sequence. And so the sequence of the DNA can be determined either manually, using that gel, from gel electrophoresis, or that sequence can also be determined autonomously using a computer, on what's known as a chromatogram, which is basically this plot that you see over here on the right. And so, the way that we are going to focus on determining the sequence is going to be using the gel. And so the gel that you see down below right here can actually be read backwards from bottom to top, and you read the gel across all lanes in order to reveal the complementary DNA sequence from 5 prime to 3 prime. And so I'll show you what I mean by this down below in this image. And so, here in the example, it's saying to determine the mystery DNA sequence by analyzing the gel electrophoresis results from dideoxy sequencing. And so notice over here in this gel, again, we have each of these lanes is going to contain a different chain termination PCR reaction from previous. And so that means that each of them is going to be ending with a different nucleotide. The ones with c here end with the nucleotide c, the ones with t end with the nucleotide t, and so on. The a's end with the a's, and the g's are going to end with the g's.
The shortest fragments are going to represent the fragments closest to the 5 prime end of the PCR product. And so that's why we want to reveal the sequence from 5 prime to 3 prime; then we need to start at the bottom. And so you read the gel backwards, and notice that the band that is at the very bottom, closest to the bottom, is the one that is highlighted right here in lane t. And so that means that this first nucleotide is going to be a t, and we can go ahead and put that here, in this position, as the first nucleotide. Then, reading the gel backwards, the next one that's closest to the bottom is the yellow one, and so notice that we're looking across all lanes here. The yellow one here is going to represent a g nucleotide, so that's going to be the next nucleotide, g. Then the next one at the bottom here is an a. So we would put an a here. Then we have a c and another c. So we get 2 back-to-back c's. Then, we have a t, an a. And then last but not least, we have a g, in the final position towards the 3 prime end. And so you can see that the sequence has been revealed by reading this gel backwards, from bottom to top starting here at these positions and working in this direction. And this is revealing it from 5 prime to 3 prime end. So then next what we need to realize is now that we've revealed the complementary DNA sequence, which is the sequence of the PCR products, In order to reveal the mystery DNA sequence, we need to remember that the complementary DNA sequence is going to be complementary to the mystery DNA sequence. And so that means that we would need to use our complementary base pairing rules to figure out the sequence of the mystery DNA.
And so recall that t always base pairs with a on the opposite strand, so we have an a here. G's always base pair with c's. A's always base pair with t's, c's with g's, c's with g's, t's with a's, a's with t's, and g's with c's. And so what you see here is the mystery DNA sequence noticed from 3 prime to 5 prime since recall that DNA strands are going to be antiparallel with respect to one another when they are complementary base pairing. And so, here we've revealed the sequence of the mystery DNA. And so, you can see here how dideoxy sequencing and analyzing the gel backwards can be used to reveal the sequence. And so, once again, if the gel is not going to be analyzed manually, another way to analyze the DNA sequence is by using a computer, and the computer can generate a chromatogram, which is a plot that looks something like this. And the chromatogram is also going to reveal the sequence. And so that's an alternative method of revealing the sequence. But this here concludes our brief lesson on how to determine the DNA sequence from the gel using dideoxy sequencing. And we'll be able to get some practice applying these concepts as we move forward in our course. So I'll see you all in our next video.
According to the gel below, which of the following is the correct sequence on the unknown DNA molecule?
Dideoxy sequencing is also known as chain termination sequencing because:
The final step in a Sanger DNA sequencing reaction is to run the DNA fragments on a gel. What purpose does this serve?
Do you want more practice?
More setsHere’s what students ask on this topic:
What is dideoxy sequencing and how does it work?
Dideoxy sequencing, also known as Sanger sequencing, is a DNA sequencing method that uses dideoxynucleotides (ddNTPs) as chain terminators. The process begins with the preparation of a reaction mixture containing the template DNA, DNA polymerase, DNA primers, normal deoxyribonucleotides (dNTPs), and a small amount of one type of ddNTP. During DNA synthesis, the incorporation of a ddNTP terminates the elongation of the DNA strand because ddNTPs lack a 3' hydroxyl group necessary for forming a phosphodiester bond. This results in a collection of DNA fragments of varying lengths, each ending with a ddNTP. These fragments are then separated by size using gel electrophoresis, and the DNA sequence is determined by analyzing the pattern of terminated fragments.
 Created using AI
Created using AIWhat are the key components required for dideoxy sequencing?
The key components required for dideoxy sequencing include:
- Template DNA: The unknown DNA sequence to be determined.
- DNA Polymerase: An enzyme that synthesizes new DNA strands.
- DNA Primers: Short sequences that anneal to the template DNA and initiate DNA synthesis.
- Deoxyribonucleotides (dNTPs): The normal nucleotides (dATP, dTTP, dGTP, dCTP) used in DNA synthesis.
- Dideoxynucleotides (ddNTPs): Modified nucleotides (ddATP, ddTTP, ddGTP, ddCTP) that terminate DNA synthesis when incorporated.
These components work together in a chain termination PCR to produce DNA fragments of varying lengths, which are then analyzed to determine the DNA sequence.
 Created using AI
Created using AIHow are DNA fragments separated and analyzed in dideoxy sequencing?
In dideoxy sequencing, DNA fragments are separated by size using gel electrophoresis. The fragments, produced by chain termination PCR, are loaded into wells at the top of a gel matrix. An electric current is applied, causing the negatively charged DNA fragments to migrate towards the positive electrode. Smaller fragments move faster and travel further than larger ones. After electrophoresis, the gel is read from bottom to top to determine the sequence of the DNA. Each lane corresponds to a different ddNTP, and the position of the bands indicates the sequence of the complementary DNA strand. This sequence can be manually read or analyzed using a computer-generated chromatogram.
 Created using AI
Created using AIWhat is the role of ddNTPs in dideoxy sequencing?
Dideoxynucleotides (ddNTPs) play a crucial role in dideoxy sequencing by acting as chain terminators. Unlike normal deoxyribonucleotides (dNTPs), ddNTPs lack a 3' hydroxyl group, which is essential for forming a phosphodiester bond with the next nucleotide. When a ddNTP is incorporated into a growing DNA strand during synthesis, it prevents further elongation of the strand. This results in the production of DNA fragments of varying lengths, each ending with a ddNTP. By analyzing these terminated fragments, the sequence of the template DNA can be determined.
 Created using AI
Created using AIHow is the DNA sequence determined from a gel in dideoxy sequencing?
To determine the DNA sequence from a gel in dideoxy sequencing, the fragments from chain termination PCR are separated by size using gel electrophoresis. The gel is read from bottom to top, across all lanes, to reveal the sequence of the complementary DNA strand from 5' to 3'. Each lane corresponds to a different ddNTP, and the position of the bands indicates the specific nucleotide at each position. The shortest fragments represent the 5' end of the sequence. By reading the gel in this manner, the sequence of the unknown template DNA can be deduced. Alternatively, a computer-generated chromatogram can be used for automated sequence determination.
 Created using AI
Created using AI