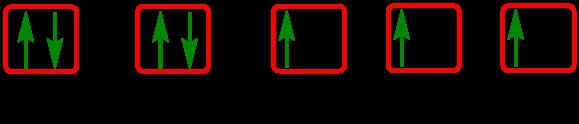
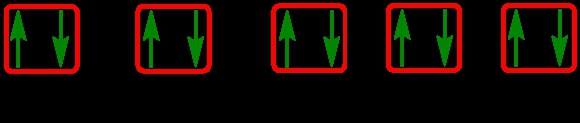
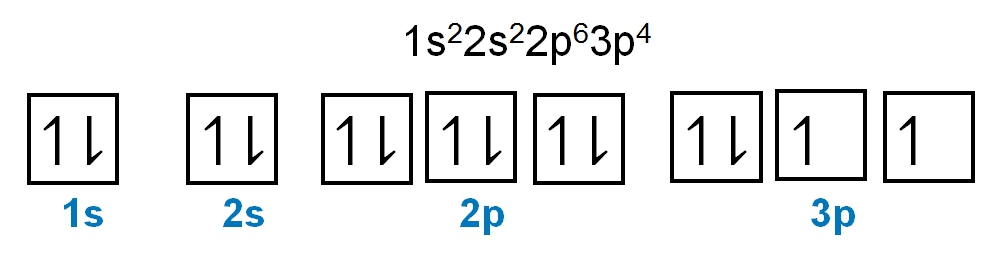
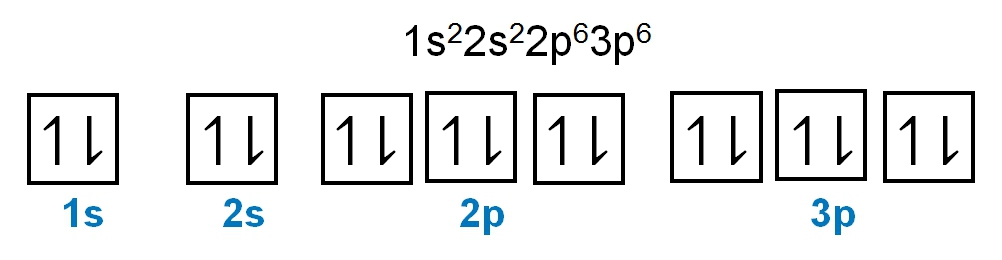
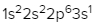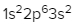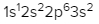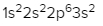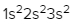As we stated earlier, the periodic law influences the electron arrangements of the elements, and the electron orbital diagrams are the visual representation of electrons within orbitals. Now, we're going to discuss what are called degenerate orbitals. These are electrons in the same set of orbitals having the same energy, and they're filled using Hund's rule. Now Hund's rule dictates that these degenerate orbitals are first half-filled before being completely filled. So if we take a look here, we have our s sublevel or s subshell. "S" can hold a maximum of 2 electrons. It has one orbital. Within that orbital, we have 2 electrons, one spins up, and one spins down. This would mean that the s sublevel can hold a maximum of 2 electrons. For the p sublevel, we have 3 orbitals. Following Hund's rule, we would half-fill them first. So we proceed as follows: up, up, up for each; since each orbital can hold a maximum of 2 electrons, we then come back around: down, down, down. Thus, the p sublevel holds a maximum of 6 electrons. For "d", we have 5 orbitals. Hund's rule states we should half-fill them first, since they're all d set of orbitals and they all have similar energy. So then we come back around: down, down, down, down, down for a total of 10 electrons. And finally, the f sublevel has 7 of these orbitals, half-filled again according to Hund's rule. Upon completely filling them, we get a total of 14 electrons. Just remember, the periodic law influences the electron arrangement of elements, and it's these orbital diagrams that depict the visual representation of electrons within any given orbital, based on subshell level or subshell letter. Keep in mind, "s" can hold a maximum of 2 electrons, "p" can hold up to 6, "d" can hold up to 10, and "f" can hold up to 14.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity16m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 15m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
The Electron Configuration (Simplified) - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIThe periodic law dictates electron arrangements, visualized through orbital diagrams. Degenerate orbitals, filled according to Hund's rule, illustrate that s, p, d, and f sublevels can hold 2, 6, 10, and 14 electrons, respectively. The Aufbau principle guides the electron configuration, filling lower energy orbitals first. Understanding paired and unpaired electrons is crucial; paired electrons occupy orbitals with opposite spins, while unpaired electrons exist alone in an orbital. This knowledge is essential for predicting chemical behavior and reactivity in various contexts, including acid-base reactions and electron transfer processes.
The electron configuration of an element is the distribution of its electrons within atomic orbitals.
The Electron Configuration
The Electron Configuration (Simplified) Concept 1
Video transcript
The Electron Configuration (Simplified) Example 1
Video transcript
It says we need to properly fill in the orbitals of an atom that possesses 8 electrons within its d set of orbitals. Now we know that these are d set of orbitals because there are 5 of them. We have to fill in 8 electrons. Remember, since they're all the same set of orbital types being d, they all have the same energy and therefore are degenerate. We would half-fill them based on Hund's rule. So we go up, up, up, up, up for our first 5. We need to fill in 8, so come back around. Down, down, down. So we stop there because again we only have 8 electrons to fill in, so here we don't need to completely fill in the last 2. They'll be left just 1 electron in each. So this would be the way to properly fill in these d set of orbitals that have 8 total electrons.
Which electron configuration represents a violation of Hund's Rule?
The Electron Configuration (Simplified) Concept 2
Video transcript
When writing the electron configuration of an element, it's important that it represents the distribution of electrons from s1 to s2 to p2 and so on within orbitals using the Aufbau principle. Now, with the Aufbau principle, it says starting with s1, we're going to have electrons filling lower energy orbitals before moving on to higher energy orbitals. We begin with s1 when we're doing the full ground state electron configuration of an element or an ion. To help us with this, you could take the Aufbau diagram approach. We start out with s1, then we move on, loop back around to s2, then we loop back around to p2, then to s3, then we loop back around to p3, and then s4, then continuously loop back around to d3, to p4, to s5. Now, this is our Aufbau diagram. In the Aufbau diagram we have here s1 all the way down to s8. Then we have p2 to p3 to p7. Then we have d3 to d6, and then we have f4 and f5. Another way we can look at determining the electron configuration has to do more with the periodic table. So, if you click on the next video, let's reimagine what the periodic table will look like when dealing with the electron configuration of elements and ions.
The Electron Configuration (Simplified) Concept 3
Video transcript
So here if we reimagine the periodic table, we'll see it in this depiction. Now realize here we have blue, yellow, purple, and red sectors. Now, each of these is called a block s. The s block is what's in blue, this is the p block, the d block, and the f block. Remember the s sublevel has 1 orbital and that one orbital can hold a maximum of 2 electrons, which is why the s block is these 2 columns, to represent the 2 maximum electrons that the s sublevel can hold. The p sublevel has 3 orbitals, right, and each one again can hold a maximum of 2 electrons. So, p theoretically can hold a maximum of 6 electrons. That's why the p block has 1, 2, 3, 4, 5, and 6 slots for it. D can have up to 10 electrons because it has 5 orbitals, and if you count you'll see in here there are 10 spots. And then for the f sublevels, they can hold up to 14 electrons, and if you were to count these rows in red, you'd see they come out to 14. Now, realize here that in this periodic table, the first slot here which represents hydrogen starts off our electron configuration as s1. Then as we move to the next one we add another electron, so helium is s2. Then when we get to the 2nd row, since we're in the 2nd row, we now have 2. This is still the s block, so this is s2 s1, and it continues onward and onward. Over here, we'd go s2, s2 p1. So following this pattern, this would be 3s, 4s, 5s, 6s, and 7s. And then here, this would be 3p4p5p6p7p. When we go to the d block, realize that it's gonna drop down by 1. So here this is 4s, but then when we go to d block, it drops down by 1 number, so now it's d3. This would be d4, d5, and d6. Now, notice how this number goes from 57 to 72, that's because 58 to 71 are here. Remember this red line here says that this entire red row exists between 57 and 72. And then we go between 89 and 104 because 90 to 103 is down here. These are your f blocks. They also drop down by one number. So this is f4 and f5. So basically, if you can reimagine the periodic table in this fashion, you can use it to figure out the electron configuration of any element or ion given to you. So we're going to put this periodic table to use in order to do future electron configurations.
The Electron Configuration (Simplified) Example 2
Video transcript
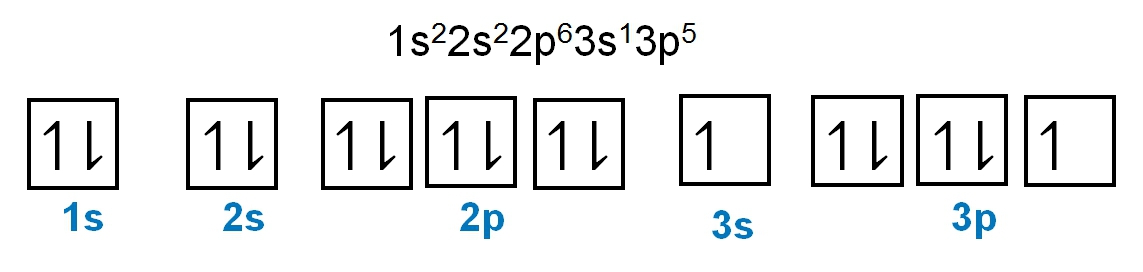
To help us with this example question, I decided to leave this periodic table up so we can see how to best use it. Here it says write the ground state electron configuration for the following element. Here we're dealing with fluorine and they tell us that it has an atomic number of 9, which means it has 9 electrons. On this periodic table, we find fluorine right here. Ground state means that we're going to start out with 1s orbital and work our way up to fluorine. So we're going to count to fluorine. So we'd say s12, s12, s22 because of 1, 2 slots. And then we have to count to f, so that would be p2, we're in the 2p row. And how many slots do we have to count to get to fluorine? 1, 2, 3, 4, 5. p25. So here, this would be the ground state electron configuration of the fluorine atom. And realize here that these are the number of electrons. So when you add them up, 2 plus 2 plus 5, that gives me 9 electrons, which is related to the atomic number here of fluorine. Remember, when an element is neutral, its atomic number tells us both the number of protons and the number of electrons. So again, rely on this depiction of the periodic table to help guide you to the right electron configuration of any element or ion given to you.
Which electron configuration represents a violation of the Auf Bau Principle?
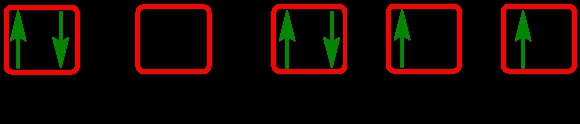
Identify the element with the given electron orbital diagram.

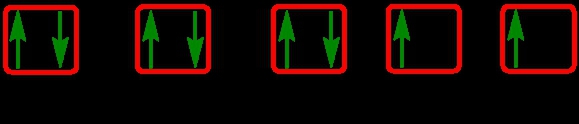
Write the electron configuration and electron orbital diagram for the following element:
Sulfur (Z = 16)
Write the ground state electron configuration for the following element:
Magnesium (Z = 12)
The Electron Configuration (Simplified) Concept 4
Video transcript
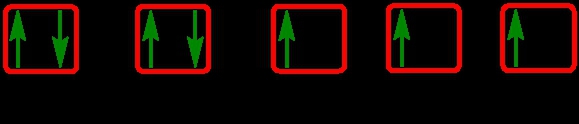
Recall an orbital can hold a maximum of 2 electrons that pair up with opposite spins. One spins up and one spins down. Now with this, we have the ideas of unpaired and paired electrons. An unpaired electron is when an orbital contains 1 electron with its own spin. So an example of an unpaired electron would be here. This orbital has only 1 electron in it pointing up. A paired electron is when an orbital contains 2 electrons, each with its own spin; so, one spins up and one spins down. So this would be a paired up orbital. But now, if we're looking at an entire electron orbital diagram, we can apply this idea of unpaired and paired electrons. The image to the left represents an unpaired electron orbital diagram because there is at least 1 orbital with an electron by itself. So as long as at least one of the orbitals has one electron, the whole thing is considered an unpaired electron orbital diagram. Now, with paired, every single electron is paired up. Every single orbital we can see has a partner, and they have opposite spins. So, keep this in mind when you're doing electron orbital diagrams. Are any of the electrons not paired up? If so, you would say that's an unpaired electron orbital diagram that's being displayed.
The Electron Configuration (Simplified) Example 3
Video transcript
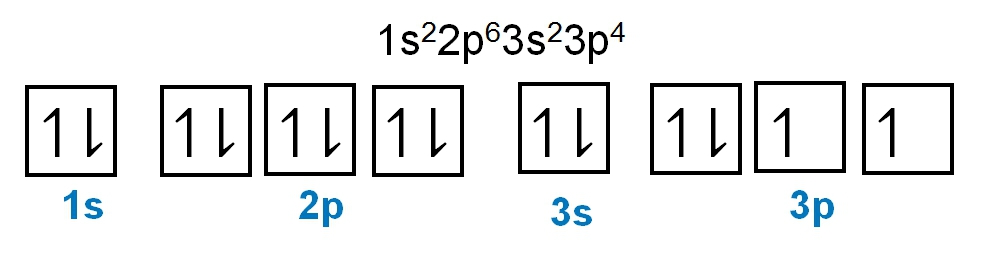
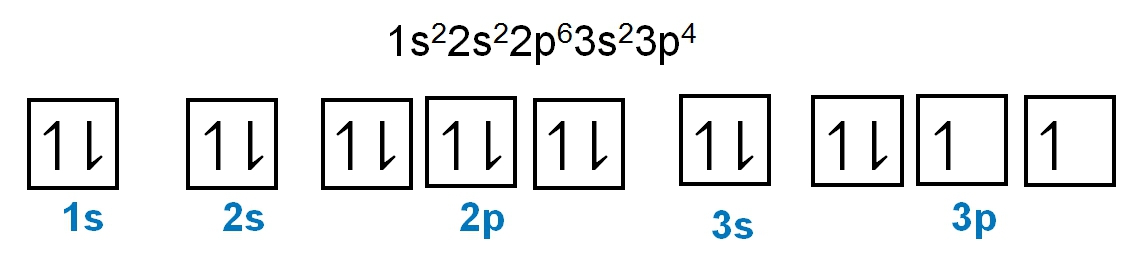
Here it says to determine the number of unpaired electrons in vanadium. So vanadium is V and it has an atomic number of 23. If we were to write out its electron configuration, we get s1 2, s2 2, p2 6, s3 2, p3 6, s4 2, d3 3. Now, Jules, do I have to look through every single one of these to find unpaired electrons? No. Just remember that s orbitals can hold a maximum of 2 electrons. And if you're hitting your maximum, that means all the electrons there are paired up. P, remember, can hold up to 6, so all those are reaching their maximum. D though, d can hold up to 10 electrons. So if it isn't hitting its maximum number of electrons, we got to check it out. So here we're gonna look at d3, and remember d has 5 sets of orbitals. Following Hund's rule, we'd half fill them because they have the same energy and there are 3 of them. Right? So we go up, up, up. There's no more electrons we can add because there are only 3 electrons here in 3d. And if we look, all 3 of them are unpaired, all of them are by themselves. That means that the answer here would have to be option D. There are 3 unpaired electrons within the vanadium element.
Which of the following atoms has the most unpaired electrons?
Here’s what students ask on this topic:
What is the Aufbau principle and how does it determine electron configuration?
The Aufbau principle states that electrons fill lower energy orbitals before moving to higher energy orbitals. This principle helps determine the electron configuration of an element by starting with the 1s orbital and progressing through 2s, 2p, 3s, and so on. The order of filling is guided by the Aufbau diagram, which shows the sequence of orbitals based on their energy levels. For example, after filling the 3p orbital, electrons will fill the 4s orbital before the 3d orbital. This systematic approach ensures that the electron configuration reflects the most stable arrangement of electrons in an atom.
 Created using AI
Created using AIWhat are degenerate orbitals and how are they filled according to Hund's rule?
Degenerate orbitals are orbitals within the same subshell that have the same energy level. According to Hund's rule, these orbitals are first half-filled with electrons before any orbital is completely filled. This means that if you have three degenerate p orbitals, each will get one electron before any of them gets a second electron. This minimizes electron-electron repulsion and stabilizes the atom. For example, in the p subshell, the three orbitals will be filled as follows: ↑, ↑, ↑, then ↓, ↓, ↓, resulting in a maximum of 6 electrons.
 Created using AI
Created using AIHow do you determine the electron configuration of an element using the periodic table?
The periodic table can be divided into blocks (s, p, d, f) that correspond to the subshells. The s block includes the first two columns, the p block includes the last six columns, the d block includes the transition metals, and the f block includes the lanthanides and actinides. To determine the electron configuration, start at hydrogen (1s1) and move across the table, adding electrons according to the block and period. For example, carbon (C) is in the second period and the p block, so its configuration is 1s2 2s2 2p2.
 Created using AI
Created using AIWhat is the difference between paired and unpaired electrons in an orbital diagram?
In an orbital diagram, paired electrons are two electrons occupying the same orbital with opposite spins (one spins up, one spins down). Unpaired electrons are single electrons in an orbital without a partner. For example, in the nitrogen atom (1s2 2s2 2p3), the 2p orbitals will have three unpaired electrons (↑, ↑, ↑). Paired electrons contribute to the stability of the atom, while unpaired electrons can influence the atom's magnetic properties and reactivity.
 Created using AI
Created using AIHow does Hund's rule apply to the filling of d and f orbitals?
Hund's rule states that electrons will fill degenerate orbitals singly before pairing up. For d orbitals, which have five degenerate orbitals, each orbital will get one electron before any orbital gets a second electron. This results in a maximum of 10 electrons (↑, ↑, ↑, ↑, ↑, then ↓, ↓, ↓, ↓, ↓). For f orbitals, which have seven degenerate orbitals, each orbital will get one electron before any gets a second, resulting in a maximum of 14 electrons (↑, ↑, ↑, ↑, ↑, ↑, ↑, then ↓, ↓, ↓, ↓, ↓, ↓, ↓).
 Created using AI
Created using AI