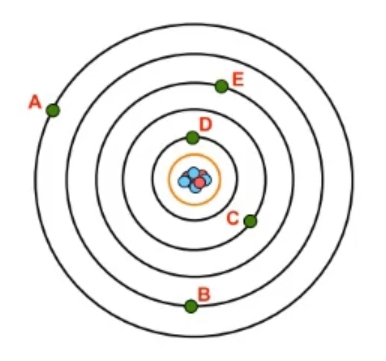
Now recall a shell, which uses the variable \( n \), is a grouping of electrons surrounding the nucleus that ties into their potential energy, so their energy of position. Now we're gonna say as the value of \( n \) increases, then both the size and energy level of an atomic orbital will increase. And we're going to say here that the energy levels, also the shell numbers of an atom can be tied to the periods or rows of the periodic table. So here we have an atom. This atom has its nucleus, and we can see that I have given it 7 shells. Right? Remember, each black orbital represents a shell. 1st shell all the way to the 7th shell here. We'll realize here that the shells of an atom are directly related to the periods or row of the periodic table. So remember, your periods or rows of periodic table go from left to right. So this is row 1, so this is shell 1, shell 2, 3, 4, 5, 6, and 7. Now currently there are 7 rows in the periodic table, but what you need to understand about the periodic table is that it is dynamic. A lot of these elements found in the 7th row of the periodic table have been discovered within the past decade. A bunch of these here were formally named in the past 10 years. So theoretically, as we move to new planets, as we explore more of the earth, as our technology gets more advanced, we're gonna create and find new elements. So eventually, there's gonna be an 8th row of elements, and then a 9th row, and so on and so forth. We're only limited by our imagination and our ingenuity. The number of elements can continuously increase again as we discover them and as we create them. Now we're gonna say because of this, the limitation of the \( n \) value is that it must be an integer, remember, whole number, from 1, because the smallest number shell can be is 1, to infinity. There's an infinite number of possible shells. Again, we're only limited by our resources, our imagination, and our ingenuity. We can create new elements with 10 shells, 12 shells, and so on. So keep in mind the relationship between rows of the periodic table and shell numbers of an atom and realize the limitation of it is that it can be any number from 1 to infinity.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity16m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 15m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Electronic Structure: Shells: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIIn atomic theory, the principal quantum number (n) indicates the energy level and size of an atomic orbital, with values ranging from 1 to infinity. Each period in the periodic table corresponds to a shell, with currently seven known rows. As technology advances, new elements may be discovered, potentially leading to additional rows. Understanding the relationship between shell numbers and periodic table rows is crucial for grasping atomic structure and the dynamic nature of elements.
Shell number gives both the size and energy of the shell.
Electronic Structure:Shells
Electronic Structure: Shells Concept 1
Video transcript
Electronic Structure: Shells Example 1
Video transcript
Of the following, is a possible value for the shell of an atom? Remember, we talked about the only real limitation when it comes to the shell number of an atom is that it's connected to the rows of the periodic table, and because of that it can be any integer from 1 to infinity. So, negative 3 can't be an exact number because that's less than 1. Same thing with negative 4. It has to be a number from 1 to infinity, so 0 is out, and e is out. So n being 2, meaning the second shell of an atom, is a possible value for n. Right? So here, the only option that works is option d.
Which of the following shell number values is a possible value for the element highlighted?
Which electron possesses the lowest possible energy from the image provided?
Here’s what students ask on this topic:
What is the principal quantum number (n) and how does it relate to the energy levels of an atom?
The principal quantum number (n) is a fundamental concept in atomic theory that indicates the energy level and size of an atomic orbital. It is denoted by the variable n and can take on integer values starting from 1 and extending to infinity. As the value of n increases, both the size and energy level of the atomic orbital increase. This means that electrons in higher energy levels (higher n values) are further from the nucleus and possess more potential energy. The principal quantum number also correlates with the periods or rows of the periodic table, where each period corresponds to a specific shell number. For example, elements in the first period have electrons in the n=1 shell, while elements in the second period have electrons in the n=2 shell.
 Created using AI
Created using AIHow are the shells of an atom related to the periods of the periodic table?
The shells of an atom are directly related to the periods (rows) of the periodic table. Each period corresponds to a specific shell number, denoted by the principal quantum number (n). For instance, the first period corresponds to the n=1 shell, the second period to the n=2 shell, and so on. Currently, there are seven known periods in the periodic table, which means there are seven known shells. As new elements are discovered or created, additional periods (and thus additional shells) may be added. This relationship helps in understanding the arrangement of electrons around the nucleus and the overall structure of the atom.
 Created using AI
Created using AICan the number of shells in an atom be infinite?
Yes, theoretically, the number of shells in an atom can be infinite. The principal quantum number (n) can take on any integer value from 1 to infinity. This means that as new elements are discovered or created, additional shells can be added. Currently, we know of elements with up to seven shells, corresponding to the seven periods of the periodic table. However, as technology advances and new elements are found or synthesized, it is possible to have elements with more than seven shells. The only limitation is our current resources, imagination, and ingenuity.
 Created using AI
Created using AIWhy is the periodic table considered dynamic?
The periodic table is considered dynamic because it evolves as new elements are discovered or synthesized. Over the past decade, several elements in the seventh period have been formally named and added to the table. As scientific research progresses and technology advances, it is likely that more elements will be discovered, leading to the addition of new periods and shells. This dynamic nature reflects the ongoing exploration and understanding of atomic structure and the potential for discovering new elements beyond the current known ones.
 Created using AI
Created using AIWhat is the significance of the relationship between shell numbers and periodic table rows?
The relationship between shell numbers and periodic table rows is significant because it helps in understanding the arrangement of electrons in an atom. Each row (period) of the periodic table corresponds to a specific shell number, denoted by the principal quantum number (n). For example, elements in the first row have electrons in the n=1 shell, while elements in the second row have electrons in the n=2 shell. This relationship provides a systematic way to determine the electron configuration of elements and predict their chemical properties. It also highlights the periodic nature of elements, where elements in the same column (group) have similar valence electron configurations and exhibit similar chemical behavior.
 Created using AI
Created using AI