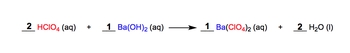
A molecular equation shows the intact compounds instead of their dissociated ionic forms. When we talk about a molecular equation, we're reacting aqueous reactant 1 with aqueous reactant 2 to produce product 1 plus product 2. A good example of this is we have 2 moles of perchloric acid in their aqueous form, plus 1 mole of barium hydroxide, creating or producing 1 mole of barium perchlorate plus 2 moles of water as liquid.
Now, when it comes to molecular equations, we can define different types of molecular equations based on the products they form. In a neutralization equation, we have an aqueous acid reacting with an aqueous base, just like in the example we provided, and what we tend to form as products are some type of ionic compound plus water. As we progress to later chapters discussing more advanced acid and base reactions, we'll see that sometimes we don't form water. For now, just realize that in a basic acid-base neutralization equation, we'll produce water and ionic salt as products.
Now, in a gas evolution equation, from the name we know that a gas is involved; here we still have aqueous reactant 1 and 2 reacting, and at least one of the products produced will be a gas. So, we'll have a gas plus product 2 — product 2 itself could also be a gas, but that's not always guaranteed.
Finally, we have a precipitation equation, and from the name 'precipitation', we know that a precipitate is involved. If you've watched my solubility rules videos, you would know that a precipitate represents a solid. So, in a precipitation equation, if at least one of the products formed is a solid ionic compound. So just remember, we have our molecular equation, which tends to have aqueous reactant 1 and aqueous reactant 2 reacting to create products 1 and products 2. Based on the identities of those products, we can have different types of molecular equations.