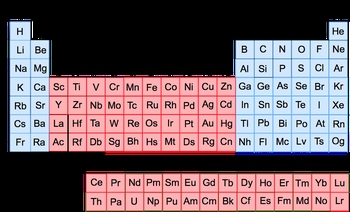
So at this point, we know that the elements in the periodic table can be organized into rows called periods and into columns called groups. Now within those groups, we can go a little bit further in our classification of these elements. We can classify them as either transition metals or representative elements. When we say transition metals, we are referring to elements found in groups 3 to 12. They're called transition metals because that name symbolizes their varying charges. For example, manganese (Mn) is a transition metal. Depending on what element it pairs with, it can have a positive charge ranging from +1 to +7. We don't have to worry about charges just yet, but realize that they're called transition metals because they have varying charges based on the elements near them. We also say that between Lanthanum (La) and Hafnium (Hf) we have this row of transition metals, and between Actinium (Ac) and Rutherfordium (Rf) we have another row of transition metals. They're still transition metals, but because they're found inside these different pairs, we call them the inner transition metals.
Besides that, we sometimes refer to the transition metals as Group B elements. What does that mean? Well, that means we can further classify or label the groups 3 to 12. So Group 3, we can now label as Group 3B if we want, 4 would be 4B, 5 would be 5B, 6B, and 7B. Here's the thing, Groups 8, 9, and 10 are all grouped together as Group 8B, and then Group 11 is 1B and Group 12 is 2B. It's a little bit weird that it goes in this order, but again, knowing why exactly goes beyond the scope of this course, so don't worry about it. What's important is that our transition metals are Group B elements. They're called transition metals because later on, we'll see that some of them have multiple charges. Representative elements, however, are the remaining elements not found in Groups 3 to 12. So we're talking about Group 12 and then Groups 13 to 18. They are sometimes referred to as our Group A elements or our main group elements. So if they're talking about Group A elements, main group elements, all that means is we're dealing with the representative elements. Group 1 can be classified as Group 1A, 2 would be 2A, Group 13 would be 3A, and it will go all the way up to 8A. So this is just another way of looking at a particular group of the periodic table and this would be 8B, 8A. Just remember when we talk about our groups we can further break it down into transition metals and representative elements. Our representative elements are sometimes referred to as our Group A elements or main group elements, and our transition metal elements are referred to as our Group B elements.