Ions are created from losing or gaining electrons by elements in order to become like the noble gases. Now we're going to say that a metal tends to lose electrons to become a positively charged ion called a cation, and nonmetals tend to gain electrons to become a negatively charged ion called an anion. Again, elements do this in order to obtain a stable electron arrangement like the noble gases. So that's the real reason behind them gaining or losing electrons. Now what we need to realize is that associated with this losing and gaining of electrons, we have the term isoelectronic. Isoelectronic just means elements have the same number of electrons. Alright. So just keep in mind when we're forming ions, we're either gaining electrons or losing electrons.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity16m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 15m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Ions (Simplified) - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIIons form when elements lose or gain electrons to achieve a stable electron configuration similar to noble gases. Metals typically lose electrons, becoming positively charged cations, while nonmetals gain electrons, forming negatively charged anions. For example, boron loses three electrons to become a cation with a charge of 3+, while oxygen gains two electrons, resulting in a charge of 2-. The term isoelectronic refers to different elements having the same number of electrons, highlighting the significance of electron transfer in ion formation.
Ions represent elements that possess either a positive charge or a negative charge.
Ions and Stable Electron Arrangements
Ions (Simplified) Concept 1
Video transcript
A positively charged ion is called a cation and a negatively charged ion is called an anion.
Ions (Simplified) Concept 2
Video transcript
So when thinking of ion formation, let's take a look at boron and oxygen. Alright. So for cation formation, remember, a cation is formed from the losing of electrons. Here we're looking at Boron 11. 11, remember, it represents its mass number or atomic mass. That's the number of protons and neutrons together. 5 just gives us the number of protons within the nucleus. And if the structure is neutral, it also has 5 electrons. We can see the 5 electrons they would have. Now, the 5 electrons are orbiting the nucleus. All of a sudden, though, I decide to remove 3 of those electrons. It now only has 2 electrons remaining. It just lost 3 electrons, so as a result, boron now gets a charge of 3+. Remember, for each electron lost, we become more positive by 1. On the other side, we have our anion formation. Anion means we're gaining electrons. Here we're dealing with oxygen 16. 16 is the mass number again, that's number of protons and neutrons together, so those are found within the nucleus. What we're more concerned with is that its atomic number is 8, which means it has 8 electrons when it's neutral. So here we see 1, 2, 3, 4, 5, 6, 7, and 8. All of a sudden, though, I decide to add 2 additional electrons. So here we've added these 2 additional electrons. So it has its original 8, of course, but then I've added 2 more. When you gain electrons, you gain a negative charge. Because I gained 2 electrons, oxygen's new charge is now 2 minus. So here, this is what we need to think of when we talk about cation and anion formation. Are we losing electrons or are we gaining electrons?
Ions are formed from either the loss or gain of electrons by a neutral element.
Ions (Simplified) Example 1
Video transcript
So here in this example question, it states, determine the number of protons, neutrons, and electrons for the following cation. Now it's a cation because it possesses a positive charge, that positive charge being 3+. Now, when looking at the other numbers, we know that this 13 here represents our atomic number, which uses the variable z. Remember, the atomic number gives us the number of protons, and because it's 13, that means we have 13 protons. That means that option D cannot be an answer because here we do not have 27 protons. Next, we have the number above the 13 and 27. Remember, that is your mass number, which uses the variable a. Your mass number gives you the number of protons plus neutrons within the element or ion. From this information, we know that the number of neutrons would equal a − z. So it's the mass number minus the atomic number. So that would be 27 minus 13, which would give us 14 neutrons. So, so far, options B and C can be the only correct choices because in A, we do not have 27 neutrons. Now the charge is 3+. 3+ means that you have lost electrons. It means you have lost 3 electrons. When aluminum is neutral, has no charge, it has an equal number of protons and electrons. Okay. But now we've lost 3 electrons, so what does that mean? That means we have 10 electrons remaining. So the answer would have to be C here, and if you want to double check that, you can just say we have 13 protons and 10 electrons, so we know that would be plus 13 plus minus 10, which would give us +3 as an answer. That proves that our charge should be +3 here. So out of all the choices present, only option C gives the correct number for each of the subatomic particles.
Give the correct number of protons, neutrons and electrons for the following isotope: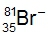
In which pair are the two species both isoelectronic and isotopic?
One isotope of a metallic element has a mass number of 65 and 35 neutrons in the nucleus. The cation that this atom forms has 28 electrons. What is the symbol of the cation?
Which of the following is the symbol for the ion with a +4 charge, 30 neutrons and 21 electrons?
Fill in the gaps for the following table.

Problem Transcript
Here’s what students ask on this topic:
What is the difference between a cation and an anion?
A cation is a positively charged ion formed when a metal loses electrons. For example, when boron (B) loses three electrons, it becomes a cation with a charge of 3+ (B3+). An anion, on the other hand, is a negatively charged ion formed when a nonmetal gains electrons. For instance, when oxygen (O) gains two electrons, it becomes an anion with a charge of 2- (O2-). The key difference lies in the type of charge and the process of electron transfer: cations lose electrons, while anions gain electrons.
 Created using AI
Created using AIWhy do elements form ions?
Elements form ions to achieve a stable electron configuration similar to that of noble gases. Noble gases have a complete valence shell, which makes them highly stable. Metals tend to lose electrons to form positively charged cations, while nonmetals gain electrons to form negatively charged anions. This electron transfer allows elements to attain a stable, lower-energy state, mimicking the electron configuration of the nearest noble gas.
 Created using AI
Created using AIWhat does isoelectronic mean in the context of ions?
Isoelectronic refers to different elements or ions that have the same number of electrons. For example, the cation Na+ (sodium ion) and the anion F- (fluoride ion) are isoelectronic because both have 10 electrons. This concept is important in understanding how different ions can have similar electron configurations, despite being different elements or having different charges.
 Created using AI
Created using AIHow does boron form a cation?
Boron (B) forms a cation by losing three electrons. In its neutral state, boron has 5 electrons. When it loses 3 electrons, it is left with 2 electrons, resulting in a positively charged ion with a charge of 3+ (B3+). This loss of electrons allows boron to achieve a more stable electron configuration.
 Created using AI
Created using AIHow does oxygen form an anion?
Oxygen (O) forms an anion by gaining two electrons. In its neutral state, oxygen has 8 electrons. When it gains 2 additional electrons, it has a total of 10 electrons, resulting in a negatively charged ion with a charge of 2- (O2-). This gain of electrons allows oxygen to achieve a more stable electron configuration.
 Created using AI
Created using AI



