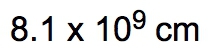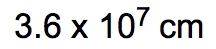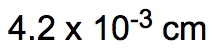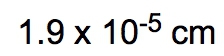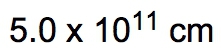So dimensional analysis is seen as a fail-proof process that allows you to convert from one unit to another. You design the problems to begin with your given amount and to finish with the end amount of your unknown. Now just follow the units to ensure the unwanted units are canceled out. In the same way that we did metric prefix conversions where we line things up on opposite levels to help them cancel out, we're going to take that and apply it here to the dimensional analysis. Now the strategy is, many conversion problems will utilize your given amount and conversion factors, and you're going to use those together to help isolate your end amount. So if we take a look here in this general format, let's say we're given 32 inches, so 32 inches is our given amount, and they're asking us to identify how many centimeters this equals. So centimeters is our end amount, what we're looking for. The conversion factor is a way of bridging these two ideas together. It's used to convert our given amount into our end amount. Now, to go from inches to centimeters means that we have to use a conversion factor that relates inches to centimeters. When we talked about conversion factors, we said that 1 inch is equal to 2.54 centimeters. I put inches here on the bottom so that they can cancel out with these inches up here on top. So you always want them on opposite levels. Doing that helps me isolate my end amount unit, which is centimeters. So then I would just multiply 32 times 2.54. Initially, I'll get 81.28 centimeters. But remember, when you're multiplying numbers together, your answer is the least number of significant figures (sig figs). 32 has 2 sig figs in it. Because, remember, when you don't have a decimal point, you move from right to left. You start counting once you get to your first non-zero number and count all the way through. So this has 2 sig figs. When you have a decimal point, you go the opposite way. Our first non-zero number is this 2. You start counting here, you go all the way through. So we have 3 sig figs. So you go with the least number of sig figs, which would be 2. So this would round to 81 centimeters as our end amount. But let's say we had to do a type of dimensional analysis with way more steps. What do we do then? Well, let's say here we are given 115 minutes. So that is our given amount, and we have to get to years. Years would be our end amount. To connect given to end, we have to utilize conversion factors. I need to cancel out minutes, so I'll put minutes here on the bottom. We know that minutes is connected to hours. We know that 1 hour has 60 minutes, and here, this will represent our first conversion factor, which I'll abbreviate that Minutes would cancel out. Now I have hours. We know that hours and days are connected. We know that one day has 24 hours. This would be my second conversion factor or CF 2. Hours cancel out. Now I have days. And then finally, we know that days to years can be connected as well. We put days on the bottom so we can cancel out with the days on top, and we know that 1 year has approximately 365 days. This is my conversion factor 3. So days cancel out, and now I'm left with years. So what we're going to have here is 115 on top multiplying with a bunch of ones, which doesn't change anything, but on the bottom, we have multiplying 60, 24, and 365. Now it's incredibly important you know how to plug this into your calculator. My suggestion when you have multiple things on the bottom multiplying is to just multiply them all together and get that sum total. When we multiply 60 times 24 times 365, their total is 525,600, and we still have the 115 on top. When we divide 115 by that total, we're going to get 2.19×10-4. We're going to say here that the number of sig figs within our final answer is based on our given information. Now our only given information was the 115. These other numbers were not given to us in any way. So we can't use them to determine the number of sig figs. Again, it's all based on the information that is presented. Look at those numbers presented to you and use those to determine the number of sig figs in your final answer. 115 has no decimal point, so we move from right to left. It has in it 3 sig figs. So our answer should have 3 sig figs, which it does. So 2.19×10-4 years would be our answer here. So everything we've learned up to this point, we're going to use in some way when it comes to dimensional analysis. And remember, our conversion factors are incredibly important because they're a way of connecting our given amount to our end amount. The whole point is to cancel out units and isolate the unit that you're looking for at the end. Now that we've done these example questions, let's move on in our discussion of dimensional analysis.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity17m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 12m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Dimensional Analysis: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIDimensional analysis is a systematic method for converting units, ensuring unwanted units cancel out. For example, converting 32 inches to centimeters involves using the conversion factor
We use Dimensional Analysis as a fail proof process to convert from one unit to another.
Dimensional Analysis
Dimensional Analysis
Video transcript
Using Dimensional Analysis, start with the given amount and obtain the end amount by using conversion factors.

Dimensional Analysis Example 1
Video transcript
Here are example states. A TA can grade 4 assignments per hour. If each assignment has 12 questions, how many questions can the TA grade in 130 minutes?
Alright. So if we're going to approach a question like this, let's look at our steps. If present, start with the given amount that is not a conversion factor. So remember, our given amount is when we have a single unit by itself that isn't tied to another. We're going to say within this question, our 130 minutes is our given amount.
Second, identify the end amount you want to isolate for your unknown. So here, this is our given amount, we have to figure out what our end amount will be. So our end amount, in this case, is questions, how many questions. Step two, write down all the conversion factors. So all our conversion factors, let's see. We have 4 assignments per hour. Remember, "per" is the word that connects different units together. So that's 4 assignments for every 1 hour. They also tell us each assignment has 12 questions. So that means one assignment is 12 questions.
The last part, find the connection between the given amount and the conversion factors in order to isolate the end amount. Let's look at the given amount. The given amount has minutes within it, but neither of the conversion factors has minutes involved. What we do have is hours. That tells me there's a conversion factor that's even before either one of these two. So conversion factor 1 actually involves us first changing minutes into hours. So we have minutes here on the bottom, hours here on top. 1 hour is 60 minutes. Minutes cancel out this way. And the reason we're doing that is because now that we have hours, we can connect it to the hours here within this conversion factor.
So that'll be my second conversion factor, bringing in the 1 hour for every 4 assignments. Now that we have hours lined up, they cancel out. Now we have assignments, and we need to get to questions. Here is our last conversion factor. Now it has assignments and questions within it, but we need assignments to cancel out. So assignments need to be here on the bottom. Right? And then we need questions, questions go here on top. And it is one assignment is 12 questions.
Remember, one of the first things we said is that conversion factors, we can flip them. They can be presented in two different ways. Here, we had to flip the initial conversion factor so that assignments can cancel one another out. They have to be on opposite levels to be able to do that. So in conversion factor 3, what I'll have left at the end is questions. So what we do now is we're going to multiply 130 times 4 times 12 divided by 60. When we do all of that, we're going to get here, as our final answer, 104 questions. So we have 104 questions as our end amount, but remember we need to worry about significant figures. 130 has 2 significant figures, 4 has 1 significant figure, 12 has 2 significant figures, 60 has 1 significant figure. So we need to go and have only 1 significant figure as our final answer. So we'd say roughly about 100 questions is what the TA could grade within 130 minutes, which is a lot of questions.
Alright. So now that we've done this example where we've set up the basic principles behind dimensional analysis, let's continue onward and do some practice questions.
If the distance between Washington, D.C. and New York City is 224.9 miles, determine the distance in centimeters.
The average human body is composed of approximately 160 fluid ounces of blood. How many quarts of blood does the average human body possess? (1 gallon = 4 quarts, 1 pint = 2 cups, 1 cup = 8 fluid ounces, 1 quart = 2 pints).
Lipitor, a pharmaceutical drug that has been shown to lower 'bad' cholesterol levels, while boosting 'good' cholesterol levels had over $12 billion in sales last year. Each pill weighs 2.5 g, which contains 4.0% of the active ingredient by mass. What mass in kg of the active ingredient is present in one bottle of 120 pills?
Your Introduction to Chemistry tutor
- Solve each of the following problems using one or more conversion factors: d. A plant fertilizer contains 15%...
- Solve each of the following problems using one or more conversion factors: c. An athlete has 15% body fat by ...
- Solve each of the following problems using one or more conversion factors: a. A container holds 0.500 qt of l...
- Solve each of the following problems using one or more conversion factors: b. What is the mass, in kilograms,...
- Solve each of the following problems using one or more conversion factors: a. Wine is 12% alcohol by volume. ...
- Using conversion factors, solve each of the following clinical problems: b. A patient needs 0.024 g of a sulf...
- Using conversion factors, solve each of the following clinical problems: a. You have used 250 L of distilled ...
- Using conversion factors, solve each of the following clinical problems: a. The physician has ordered 1.0 g o...
- Calcium citrate, Ca(C6H5O7)2(MW = 498.5 amu), is a common dietary supplement to provide calcium needed for str...
- The white blood cell concentration in normal blood is approximately 12,000 cells/mm^2 of blood. How many white...
- Obtain a package of your favorite snack food and examine the nutritional information on the label. Confirm the...
- The length of this rug is 38.4 in. and the width is 24.2 in. (2.3, 2.6) d. Calculate the area of the rug, in ...
- A shipping box has a length of 7.00 in., a width of 6.00 in., and a height of 4.00 in. (2.3, 2.6) b. What is...
- A shipping box has a length of 7.00 in., a width of 6.00 in., and a height of 4.00 in. (2.3, 2.6) a. What is...
- Gemstones are weighed in carats, where 1 carat = 200 mg exactly. What is the mass in grams of the Hope diamond...
- In France, grapes are 1.95 euros per kilogram. What is the cost of grapes, in dollars per pound, if the exchan...
- In Mexico, avocados are 48 pesos per kilogram. What is the cost, in cents, of an avocado that weighs 0.45 lb i...
- To treat a bacterial infection, a doctor orders 4 tablets of amoxicillin per day for 10 days. If each tablet c...
- a. An athlete with a body mass of 65 kg has 3.0% body fat. How many pounds of body fat does that person have? ...