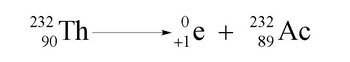Positron emission is a fascinating nuclear process where an unstable nucleus releases a positron, which is the antiparticle of the electron. While an electron carries a negative charge, a positron has a positive charge, making it essentially a "positive electron." This concept may seem unusual, but it is a key aspect of nuclear reactions.
In the context of positron emission, the term "emission" refers to the decay of the nucleus, indicating that the positron is a product of this decay process. To illustrate this, consider the element Einsteinium, specifically its isotope Einsteinium-253 (symbol: Es), which has an atomic number of 99. When this isotope undergoes positron emission, the atomic mass remains unchanged at 253, as the positron has an atomic mass of 0. However, the atomic number decreases by 1, since emitting a positron effectively transforms the element into a different one. Therefore, the new element formed is Californium (symbol: Cf), which has an atomic number of 98.
This example highlights the transformation that occurs during positron emission, showcasing how nuclear reactions can lead to the creation of new elements through the emission of particles like positrons.