Hey guys, in this new video, we're going to take a look at positron emission. So, here we're going to say positron emission occurs when an unstable nucleus emits a positron. Now, what's a positron? A positron is an antiparticle of the electron. Remember, the electron is represented by e-. A positron is the opposite of that. So, it looks like an electron, but instead of it having a negative sign, it will have the opposite sign. So, it will be a positive electron. So, a positron is considered just a positive electron. I know this is weird, but again remember, we're dealing with nuclear reactions. So, a lot of unaccustomed things that we are not used to seeing do occur. And one of them is this positron. So, we're going to say here, here's our positron. Now, because we're talking about the word emission again, emission would mean decay, which means that this positron would be a product. So, let's think of an example. Here, Einstein has his own element named after him, Einsteinium. So Einsteinium will deal with isotope 253 of Einsteinium. So, Einsteinium is ES on our periodic table. It has an atomic number of 99. We're going to emit a positron and because we're emitting a positron, let's see. So because the atomic mass is 0, the new element is still going to be 253. But because the bottom is 1, what number plus 1 gives me 99? It would have to be 98. So, here, that would just be CF. So, this would be an example of a positron decay or positron emission.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity16m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 15m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Positron Emission: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIPositron emission occurs when an unstable nucleus emits a positron, which is the antiparticle of the electron, possessing a positive charge. This process is a type of radioactive decay. For example, when the isotope Einsteinium-253 (atomic number 99) undergoes positron emission, it transforms into Californium-253 (atomic number 98), as the atomic mass remains unchanged while the atomic number decreases by one. Understanding positron emission is crucial in nuclear chemistry and applications like positron emission tomography (PET).
A positron emission or positron decay occurs when an unstable nucleus ejects a positron particle to create a new element.
Understanding Positron Emission
A positron particle is referred to as the anti-electron particle because it looks like a positively charged electron.

Positron Emission Concept 1
Video transcript
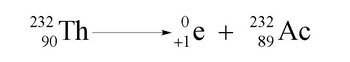
Positron Emission Example 1
Video transcript
Now, based on that, let's answer these two questions. It says, write balanced nuclear equations for each of the following positron emissions. So again, your positron will be a product. So here we're dealing with uranium 235. Uranium is U. On your periodic table, it's going to have an atomic number of 92. We emit a positron, so this is going to be:
U 235 , 92 → Pa 235 , 91 + e + 0 , 1The next one is radon which is Rn. Radon has an atomic number of 86. We emit a positron. So now, the element is still going to be 222 and 85 +1 gives me the 86. So, that's Actinium. The equation is:
Rn 222 , 86 → Ac 222 , 85 + e + 0 , 1These would be two examples of our positron emission.
Positron Emission Example 2
Video transcript
Now, following what we've learned so far about all the different types of decays and emissions out there, let's try our best to answer this one. So here we're going to say a nucleus of Thorium-225 undergoes three alpha decays, four beta decays, and a gamma emission. What is the product? So, remember Thorium has an atomic number of 90. It's going to undergo three alpha decays, and alpha decays basically produce the helium or alpha particle, plus four beta decays, so that's four electrons being emitted, plus gamma, which is 0 over 0. So, basically when you have three helium particles being emitted, that's 3×4 which is 12, 3×2 which is 6, 4×0 which is 0, 4×-1 which is -4. Again, this is not the proper way to write it. I'm just combining all the math just to make it easier for us to see what the answer is. So, we're going to say 12 here. So the new element would have to be 213 because 12 + 213 will give me the 225 I started out with. And then here, 6 plus this negative 4 gives me 2. So it would have to be 88 here because 88 + 2 will give me back the 90 I started out with. So, then we're going to say we have 88, so that is Ra, which is radium. And because we underwent a gamma emission, technically, this would be in an excited state. So, we put a little asterisk there to show that it's in an excited state. So, that's all we have to do. And this top equation is the correct way to write the equation. Remember, oops, remember I just combined everything here to make the math easier for us to see what's going on. But it's this top equation that's the correct format that your professor would be looking for. So, just remember, fundamentally, decay and emission mean the particle will be a product. Capture or absorption means it'll be a reactant. That's the key to this. And then, just remembering what's an alpha particle, a beta particle, a positron, a gamma, all those different concepts.
Here’s what students ask on this topic:
What is positron emission and how does it occur?
Positron emission is a type of radioactive decay where an unstable nucleus emits a positron. A positron is the antiparticle of the electron, possessing a positive charge. This process occurs when a proton in the nucleus is converted into a neutron, releasing a positron and a neutrino. The emitted positron is represented as . For example, when Einsteinium-253 (atomic number 99) undergoes positron emission, it transforms into Californium-253 (atomic number 98), as the atomic mass remains unchanged while the atomic number decreases by one.
 Created using AI
Created using AIWhat is the difference between a positron and an electron?
A positron is the antiparticle of the electron. While an electron has a negative charge, represented as , a positron has a positive charge, represented as . Both particles have the same mass but opposite charges. Positrons are involved in processes like positron emission, a type of radioactive decay, whereas electrons are commonly found in atomic orbitals.
 Created using AI
Created using AIWhat is an example of positron emission in nuclear reactions?
An example of positron emission in nuclear reactions is the decay of Einsteinium-253. When Einsteinium-253 (atomic number 99) undergoes positron emission, it emits a positron and transforms into Californium-253 (atomic number 98). The atomic mass remains the same at 253, but the atomic number decreases by one due to the conversion of a proton into a neutron. The reaction can be represented as: .
 Created using AI
Created using AIHow is positron emission used in medical applications?
Positron emission is used in medical applications such as Positron Emission Tomography (PET). PET scans are imaging tests that help doctors observe metabolic processes in the body. A radioactive tracer that emits positrons is introduced into the body. When these positrons encounter electrons, they annihilate, producing gamma rays. These gamma rays are detected by the PET scanner, creating detailed images of organs and tissues. PET scans are particularly useful in detecting cancer, monitoring heart conditions, and studying brain function.
 Created using AI
Created using AIWhat changes occur in the nucleus during positron emission?
During positron emission, a proton in the nucleus is converted into a neutron. This process releases a positron and a neutrino. The emitted positron is represented as . As a result, the atomic number of the element decreases by one, while the atomic mass remains unchanged. For example, when Einsteinium-253 (atomic number 99) undergoes positron emission, it transforms into Californium-253 (atomic number 98).
 Created using AI
Created using AI