Guys, in this new video, we're going to take a look at gamma emissions. So here we're going to say gamma radiation is related to the electromagnetic spectrum. Now, we're going to say here gamma rays have the highest energy and therefore they have the lowest or shortest wavelength, and then they have the highest frequency. So remember, you have to remember from electromagnetic spectrum theory, that when it comes to energy, energy and frequency are directly proportional. All that means is if one is high, the other one would be high. But when it comes to wavelength, wavelength is inversely proportional to both of them. All that means is that wavelength is the opposite of the other 2. If they're high, it's low. If it's high, they're low. So it's a complete opposite of frequency and energy, and remember, wavelength is just the distance from one wave to another wave. Where frequency is, how many waves do you get within one second. So if your distance between waves, the crest, the tops of them is very large, that means you don't get many waves in a second. But if the wavelength is very small, if the distance between them is very small, you can cram a bunch of them in within one second. Okay. So then you would say the frequency is extremely high, and the energy is high. Gamma rays have the highest behind cosmic rays. Cosmic rays would actually be a little bit higher than gamma rays. We usually don't hear about this in lecture, but in lecture, so strictly lecture, we're going to say gamma rays have the highest energy, and therefore they have the highest frequency, and therefore the lowest or shortest wavelength. If you went beyond just general chemistry, you go into physics and other, higher-level sciences, they'd start talking about cosmic rays, which are then even higher than gamma rays. But for right now, just focused on the simple electromagnetic spectrum, gamma rays are going to have the highest energy.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity17m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 12m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Gamma Emission: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIGamma rays are a form of electromagnetic radiation with the highest energy, shortest wavelength, and highest frequency. They are represented as , indicating no change in atomic mass or number during gamma emission. This process involves energy absorption, causing electrons to transition to higher energy levels. Despite having the lowest ionizing power, gamma rays possess the highest penetrating power, making them extremely hazardous to biological tissues, as even minimal exposure can be lethal.
The gamma particle does not create a new element like the other radioactive particles, but instead causes the excitation of electrons within an element.
Understanding Gamma Emission
A gamma particle has no atomic mass and no atomic number and is represented by the sign gamma.

Gamma Emission Concept 1
Video transcript
Gamma radiation is involved in the electromagnetic spectrum. Gamma rays possess the highest energy, while radio waves have lowest energy in terms of the spectrum.
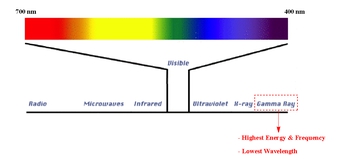
Gamma Emission Concept 2
Video transcript
Now we're going to say a gamma particle can be represented by 00 and the gamma symbol is this. Now, you're going to say because it's 00, you should realize that a gamma ray actually does not cause any change in your atomic mass or atomic number. And because of that, we usually see it happening with alpha decay or beta decay. But what's the whole purpose of gamma emission then? Well, we're going to say when it comes to gamma emission, it has to do with the absorption of energy. So here we're going to say this wavy live line represents energy, and this electron is in our first shell in our atom. So here in absorption, the electron is going to absorb that excess energy and become excited, and use that extra energy just absorbed to jump up to either a higher shell number or to a higher orbital number. So basically, if you go from 1s2 to 2s2. So you're going from the first shell to the second shell, that represents absorption. You could also go from the 3s3 to 3d3. You can script skip 3p3 altogether, and just jump up straight to 3d3. Both of these examples represent absorption. The first one represents absorption where you jump from a one shell to a higher shell, and then the 3s3 to the 3d3 represents you absorbing energy and jumping up from a lower orbital to a higher orbital within the same shell. Both of them begin with the number 3, so they're both within the third shell of your atom, but d orbitals have more energy than s orbitals. So if we solve this, we'd have 4020Ca. It undergoes a gamma emission, and we'd say that that calcium has an electron that had just absorbed, energy and it's going to become excited. So we put a little asterisk by it to show that it's in an excited state. So that will represent a gamma emission. Now, we're going to say that gamma particles, they have the lowest ionizing power, but they have the highest penetrating power. So if you're ever exposed to gamma emission, like gamma radiation, it's basically a done deal. You're not going to survive. Gamma radiation is extremely toxic to living tissue and biological systems. So any exposure to even the smallest amount of gamma radiation would completely eviscerate all the living cells and tissues within your body. So it has the lowest ionizing power, but it's still extremely dangerous.

Gamma Particles have lowest ionizing power, but are the most dangerous because of their highest penetrating power.

Gamma Emission Example 1
Video transcript
Now, if we go to this example, it says which of the following represents an element that has experienced a gamma emission? Here we have electron configurations for elements. Remember, the normal pattern is, you go from 1s to 2s to 2p to 3s to 3p4s etcetera. And remember, gamma emission involves the excitation or the absorption of energy gets you into an excited state, so you could actually have an electron skipping an entire orbital, like it's supposed to be in the 2s, but it gains so much energy it skips 2s and goes to 2p. Or you could have an electron gaining even more energy and going from 2s to 3s. So, if we look, the one that fits this excited state or this gamma emission state would have to be sodium, because we're supposed to see 1s followed by 2s, followed by 2p, followed by 3s. But what must have happened? This electron in here must have absorbed energy and therefore able to jump to a higher energy state. So the next one would be 3p. So it skipped the 3s and went straight to 3p. So this represents an element that has gained sufficient energy to become excited, which represents a gamma emission. So remember, gamma emission may not change your atomic mass or atomic number like alpha decay or beta decay, but it still does affect the atom in some way.
Here’s what students ask on this topic:
What is gamma emission and how does it affect atomic mass and number?
Gamma emission is a type of radioactive decay where an unstable nucleus releases energy in the form of gamma rays. Gamma rays are high-energy electromagnetic radiation with the highest energy, shortest wavelength, and highest frequency. During gamma emission, there is no change in the atomic mass or atomic number of the element. This is because gamma rays are represented as 0/0, indicating that they do not alter the number of protons or neutrons in the nucleus. Instead, gamma emission typically accompanies other types of decay, such as alpha or beta decay, and involves the release of excess energy from the nucleus.
 Created using AI
Created using AIHow do gamma rays compare to other forms of electromagnetic radiation in terms of energy, wavelength, and frequency?
Gamma rays are the highest energy form of electromagnetic radiation, with the shortest wavelength and highest frequency. In the electromagnetic spectrum, energy and frequency are directly proportional, meaning that as the energy of the radiation increases, so does its frequency. Conversely, wavelength is inversely proportional to both energy and frequency, so as the energy and frequency increase, the wavelength decreases. Gamma rays have higher energy and frequency than X-rays, ultraviolet light, visible light, infrared radiation, microwaves, and radio waves. This makes gamma rays extremely penetrating and hazardous to biological tissues.
 Created using AI
Created using AIWhat is the significance of gamma rays having the highest penetrating power but the lowest ionizing power?
Gamma rays have the highest penetrating power among all types of radiation, meaning they can pass through most materials, including human tissue, with ease. However, they have the lowest ionizing power, which refers to their ability to ionize atoms and molecules. Despite their low ionizing power, gamma rays are extremely dangerous because their high energy can cause significant damage to biological tissues. Even minimal exposure to gamma radiation can be lethal, as it can eviscerate living cells and tissues. This makes gamma rays a serious concern in radiation safety and protection.
 Created using AI
Created using AIHow does gamma emission relate to the absorption of energy and electron excitation?
Gamma emission is closely related to the absorption of energy and the excitation of electrons. When an atom absorbs energy, its electrons can become excited and move to higher energy levels or orbitals. For example, an electron in the 1s orbital can absorb energy and jump to the 2s orbital, or an electron in the 3s orbital can jump to the 3d orbital. This process of absorption and excitation is often followed by the release of excess energy in the form of gamma rays. The emitted gamma rays indicate that the atom has returned to a lower energy state after the electron excitation.
 Created using AI
Created using AIWhy are gamma rays considered extremely hazardous to biological tissues?
Gamma rays are considered extremely hazardous to biological tissues due to their high energy and penetrating power. They can easily pass through the human body and cause severe damage to cells and tissues. Even minimal exposure to gamma radiation can be lethal, as it can destroy living cells and disrupt biological processes. The high energy of gamma rays can break chemical bonds and ionize molecules, leading to cellular damage, mutations, and cancer. Therefore, strict safety measures are necessary to protect against gamma radiation exposure in medical, industrial, and research settings.
 Created using AI
Created using AI