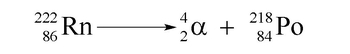
Hey, guys. In this new video, we're going to take a look at alpha decay. So remember, Rutherford talked about the 3 major types of decays. There's alpha decay, beta decay and gamma emission. Here, we have alpha decay. We're going to say alpha decay occurs when an unstable nucleus emits a particle composed of 2 protons and 2 neutrons. Now, just think about it. We say that our atomic mass equals the number of protons plus the number of neutrons. So, here our atomic mass is, we lose 2 protons and 2 neutrons, so 2 + 2 gives me 4. Your atomic mass is protons and we're going to say the alpha particle is represented by 4 for your atomic mass, over 2 your atomic number. And here we have our alpha symbol. Now, we can also say that on our periodic table, we have an element that also has an atomic mass of 4 and an atomic number of 2. That element is Helium. So, we can say that the alpha particle can also be represented by the element Helium because Helium has the same atomic mass as an alpha particle and it has the same atomic number as an alpha particle. And remember, we're using the term decay. So, decay means that this alpha particle will be a product. So, if you wanted to look at an example of this, we could think of, for example, in your periodic table, you could have polonium, when shown in your periodic table as PO. Polonium, we're going to say, let's talk about isotope-two ten.
Now remember, with these nuclear reactions, they can happen with different isotopes of an element. So, on your periodic table, we'll be doing different types of decays with different types of isotopes. So don't worry if your atomic mass on your periodic table doesn't match my atomic mass. That's because I'm dealing with a certain isotope of that element. Remember, isotopes have the same atomic number, so that's the same element, but they have a different number of neutrons. So we'll have different atomic masses. So here, polonium 210 means the atomic mass is 210. If you look on your periodic table, polonium has an atomic number, number of protons of 84. Now, we're going to undergo alpha decay. Alpha decay means we're going to spin out or emit an alpha particle. You can represent it like this or like this. Here, I'll just choose to show it as Helium. So, we're going to emit 42 Helium.
Nuclear reactions are different from regular reactions, but there are some similarities. Just like you have to have a regular chemical reaction balanced, you have to have a nuclear reaction also balanced. So here, our total atomic mass is 210. Here, we have already an atomic mass of 4. So, we need to create an element that when I add it to the 4, gives me back this mass of 210. So, the new element has to be 206, because 206 + 4 gives me 210. Also, your atomic numbers need to match on both sides. This atomic number is 2, we need it to add up to 84. So we'd say that the new element would have to have 82 because 82 plus 2 gives me 84. And what element would that be? Well, that would be lead. So, what we'd say here is that we'd say the alpha decay of polonium 210 creates a brand new element, lead-206. The Helium or the Alpha Particle is just something that we emit; it's just waste. The new element that we're concerned with is the lead-206. So, this represents an alpha decay. And it's as straightforward as that. Make sure that your atomic mass adds up on both sides. Make sure your atomic numbers add up on both sides.