Hey guys, in this brand new video, we're going to take a look at the equilibrium constant. Now, we're going to say that the variable we use to illustrate our equilibrium constant is the variable k. We're going to say k is a number equal to the ratio of products to reactants at a given temperature. Why do I say at a given temperature? Because we're going to say that temperature directly affects our equilibrium constant k. Increasing the temperature will increase our k value. Decreasing our temperature will decrease our K value. Now we're going to say it's a ratio of products to reactants, so we're going to say here that K = productsreactants. Now, we're going to say that k is important because its magnitude tells us how far to the left or to the right our chemical reactions are at a given temperature. So we're going to say if K > 1, then products are favored over reactants. Think about it. This makes sense because we said k = products over reactants. So let's say our products are 10 and our reactants are 1. K = 10, definitely greater than 1. We're going to say when products are favored over our reactants, that means we're making more products. How do we make more products? By going in the forward direction. So the forward direction would be favored. Now in the opposite way, if K < 1, so let's say in this case, products are 1 but our reactants are 10. We'd have a K < 1. So in this case, if K < 1, then reactants are favored over products, which means our reaction is heading in the reverse direction. So the reverse direction would be favored. Just remember these two differences, when k is greater than 1 and when k is less than 1. Now, what if we say k was equal to 1? We know that this is products over reactants, so that means that our products and reactants would have to be equal. Let's say they're both 10. 10 / 10 is 1. So when k equals 1, we're going to say both our reactant and our product amounts are equal to one another. Now we're going to say the equilibrium constant k takes into account all the states of matter except 2. It doesn't look at solids and it doesn't look at liquids. It ignores those 2 states for matter.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity16m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 15m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
The Equilibrium Constant - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIThe equilibrium constant, denoted as , represents the ratio of products to reactants at a specific temperature. A value of greater than 1 indicates that products are favored, while a value less than 1 suggests reactants are favored. When equals 1, products and reactants are in equal amounts. Notably, solids and liquids are excluded from this ratio, emphasizing the importance of understanding dynamic equilibrium in chemical reactions.
Equilibrium Constant K is associated with any reaction at equilibrium. Its numerical value determines if reactants or products are more greatly favored within a reaction.
The Equilibrium Constant K
The Equilibrium Constant Concept 1
Video transcript
The Equilibrium Constant K is a ratio of products to reactants. It only deals with gaseous or aqueous compounds.

The Equilibrium Constant Example 1
Video transcript
So it looks at, it only cares about really gases and also aqueous. Aqueous just means that the solvent is water, so a compound is dissolved in water. It would be aqueous. Now, knowing this, let's take a look at the following example. It says, Write the equilibrium expression for the following reaction. Here we have 2N2O5 aqueous gives us 4 NO2 aqueous plus O2 aqueous. It's important to look at the phases because remember if it's a solid or a liquid, we ignore it. Now we're going to say here k = NO24O2N2O52. The number in front of NO2 is a 4, so that 4 will become the power. So, it's going to be NO2 to the 4 times O2. O2 just has a coefficient of 1 in front of it, which we don't have to show. Divided by N2O5. Again, the coefficient in front of N2O5 is 2, so that becomes a power. So, we would say that this represents our equilibrium expression or our equilibrium equation. Same thing. Equilibrium expression, equilibrium equation. Now that we've seen this one, let's look at B. For B, we're going to say here, we're going to ignore this compound because it's a solid, and we're going to ignore this compound because it's a solid. Here we're going to say k = 1O2. But here's the thing, we're going to say we don't have any products available. But you have to put something on top. We're going to say it's equal to 1. Solids and liquids are ignored, and in place of them, we're going to put 1. We're going to say this because things such as pressure don't really affect solids and liquids like they do gases and aqueous compounds. That's why they're equal to 1 because their number is being held constant. So, we're going to replace solids and liquids with the number 1. And on the bottom, we'd have O2. Now, a different way your professor could give you this is, we know there's a one here, so this could also be or O2 inverse 1. So be aware of both types of answers. Both are correct. Both are saying the same thing. Inverse 1 just means 1 over whatever it is. For the next one, again we ignore solids. The only thing that we don't ignore is this gas. Here, k = Xe3 because of the 3 over 1. But we don't need to put the one because anything over 1 is the same exact thing. So that would be our equilibrium C. We ignore the solids and the liquids. Now that you've seen this, I want you guys to attempt to answer a question that follows this one, the practice one. Here, I want you to tell me who's greater in amount? Is it products or is it reactants? From that, you have to remember what do we say about K. When it's greater than 1, who's favored? When it's less than 1, who's favored? When it's equal to 1, who's favored? Remembering that will be a great way for you to approach this problem. Good luck, guys.
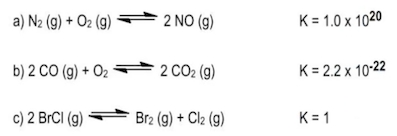
State which is greater in amount:reactants or products, based on the given equilibrium constant, K.

The decomposition of nitrogen monoxide can be achieved under high temperatures to create the products of nitrogen and oxygen gas.
6 NO(aq) ⇌ 3 N2(aq) + 3 O2(aq)
a) What is the equilibrium equation for the reaction above?
b) What is the equilibrium expression for the reverse reaction.
The equilibrium constant, K, for 2 NO (g) + O2 (g) ⇌ 2 NO2 (g) is 6.9 x 102.
What is the [NO] in an equilibrium mixture of gaseous NO, O2, and NO2 at 500 K that contains 1.5 x 10 –2 M O2 and 4.3 x 10 –3 M NO2?
Here’s what students ask on this topic:
What is the equilibrium constant (K) and how is it calculated?
The equilibrium constant, denoted as K, is a number that represents the ratio of the concentrations of products to reactants at equilibrium for a given chemical reaction at a specific temperature. It is calculated using the formula:
Here, the concentrations of the products and reactants are raised to the power of their respective coefficients in the balanced chemical equation. Note that solids and liquids are not included in the calculation of K.
 Created using AI
Created using AIHow does temperature affect the equilibrium constant (K)?
Temperature has a significant impact on the equilibrium constant (K). If the temperature increases, the value of K will also increase for endothermic reactions, meaning the reaction favors the formation of products. Conversely, for exothermic reactions, an increase in temperature will decrease the value of K, favoring the formation of reactants. This is because temperature changes affect the rates of the forward and reverse reactions differently, shifting the equilibrium position.
 Created using AI
Created using AIWhat does it mean if the equilibrium constant (K) is greater than 1?
If the equilibrium constant (K) is greater than 1, it indicates that the concentration of products is greater than the concentration of reactants at equilibrium. This means that the forward reaction is favored, and the reaction tends to produce more products. For example, if K = 10, the products are ten times more concentrated than the reactants, suggesting a strong tendency towards product formation.
 Created using AI
Created using AIWhy are solids and liquids excluded from the equilibrium constant (K) expression?
Solids and liquids are excluded from the equilibrium constant (K) expression because their concentrations do not change during the reaction. The concentration of a pure solid or liquid is constant and does not affect the equilibrium position. Therefore, only the concentrations of gases and aqueous solutions are included in the calculation of K, as these can vary and influence the equilibrium state.
 Created using AI
Created using AIWhat does it mean if the equilibrium constant (K) is less than 1?
If the equilibrium constant (K) is less than 1, it indicates that the concentration of reactants is greater than the concentration of products at equilibrium. This means that the reverse reaction is favored, and the reaction tends to produce more reactants. For example, if K = 0.1, the reactants are ten times more concentrated than the products, suggesting a strong tendency towards reactant formation.
 Created using AI
Created using AIYour Introduction to Chemistry tutor
- Do the following reactions favor reactants or products at equilibrium? Give relative concentrations at equilib...
- Do the following reactions favor reactants or products at equilibrium? Give relative concentrations at equilib...
- The following diagrams represent two similar reactions that have achieved equilibrium: <.> Calculate th...
- The following diagrams represent two similar reactions that have achieved equilibrium: <.> Write the ex...
- Write the equilibrium constant expressions for the following reactions: 2 CO(g) + O2(g) ⇌ 2 CO2(g)
- Write the equilibrium constant expressions for the following reactions. C(s) + H2O(g) ⇌ CO(g) + H2(g)
- Use your answer from Problem 7.53 to calculate the following: [N2O4] at equilibrium when [NO2] = 0.0250 mol/L
- Use your answer from Problem 7.54 to calculate the following: [O2] at equilibrium when [CO2] = 0.18 mol/L and...
- Oxygen can be converted into ozone by the action of lightning or electric sparks: 3 O2(g) ⇌ 2 O3(g) For this ...
- Magnetite, an iron ore with formula Fe3O4, can be reduced by treatment with hydrogen to yield iron metal and w...
- Ammonia reacts slowly in air to produce nitrogen monoxide and water vapor: NH3(g) + O2(g) ⇌ NO(g) + H2O(g) +...