Welcome back, guys. In this new video, we're going to take a closer look at solubility. Now, what we should realize is that solubility can have two other names associated with it. Solubility is also called concentration. So if they say concentration, it's the same thing as solubility. Another name for concentration or solubility would be molarity. Molarity is moles of solute per liter of solution. Solubility is how much solute can we dissolve in our solvent. They're both basically saying the same exact thing. Now, when we dissolve a solid, what's going to happen is ions are going to leave that solid and basically dissolve within the solvent. And what we should realize is that there's basically a threshold, a limit to how much solute we can dissolve into something. After a certain point, we can try and try and try and add more and more solid, but none of it will dissolve because the water, the liquid has reached its maximum amount of dissolving. So we should realize that when it comes to this there are different terms we need to be familiar with.
We're going to say, in a saturated solution, the maximum amount of dissolved solute is present in the solvent. So what I mean by this is let's say we have a bucket of water. And let's say that this bucket of water can only dissolve up to 100 grams of solute. Let's say I take 102 grams of solute and I dump it into that bucket. Now, the bucket will dissolve the 100 grams, no problem. But those extra 2 grams that I have are not going to dissolve. They're going to remain as solids clumped up at the bottom of the bucket of water. We're going to say that this solution has reached its maximum amount of solute. We're going to say that this solution is saturated. So remember, saturated means that the solution, the solvent has reached its maximum amount in grams and moles, whatever of the amount of solute it can dissolve successfully.
Now, in an unsaturated solution, additional amounts of solute can be further dissolved in the solvent. So we have our same bucket of water. We say that it can dissolve 100 grams. But let's say I take 90 grams of solute and I dump it in there. All 90 grams will completely dissolve, and we still have room to dissolve 10 more grams. We're going to say that this solution is unsaturated. It still has room for it to dissolve more solvent. Okay, It'll successfully dissolve the 90 but we'll still have room for 10 more.
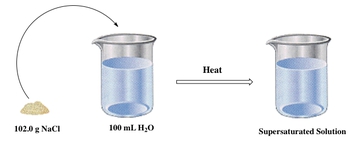
Now, finally, in a supersaturated solution, more than the equilibrium concentration of solute has been dissolved. What this means is again I have my bucket of water and somehow I get this bucket even though it's supposed to only dissolve 100, I get it to dissolve those extra 2 grams I talked about earlier. And we're going to say that this solution is supersaturated. It's gone beyond its limit. Now, you might ask, How do we accomplish this? Well, what you're going to say here is to do this you'd have to apply heat. Heat is needed to do this. If you supply heat to it, you'll be able to dissolve beyond your maximum. But here's the thing, supersaturated solutions are very unstable. So the moment that I take away the heat from the solution, the extra 2 that I dissolved is going to resolidify and form a precipitate or what we call a solid on the bottom. Okay? So remember, heat causes us to create a supersaturated solution. I can dissolve 102 grams. All 102 of it dissolves completely. Once I take away my heat, the extra 2 grams that I have here are going to form a precipitate. They're going to what we say recrystallize. They're going to recrystallize at the bottom of my bucket of water. So saturated, unsaturated, supersaturated. All of them deal with solubility. How much solute can I dissolve? All of them deal with molarity. So remember, solubility, concentration, molarity, all the same thing.