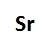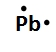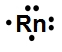Now, Lewis dot symbols, sometimes referred to as electron dot diagrams, are diagrams that represent the valence electrons of an atom or ion. When we talk about valence electrons, we look at it in terms of main group elements versus transition metals. For main group elements, the group elements that are groups 1A to 8A, the number of valence electrons that they possess is equal to their group number. So, for example, if we're looking at aluminum, aluminum is in group 3A, so it would have 3 valence electrons. For transition metals, it's a bit different. For them, the number of valence electrons equals their s+d electrons. So just remember, when we're looking at an element and trying to determine its number of valence electrons, you first have to look at it as being either a main group element or a transition metal.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity17m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 12m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Lewis Dot Symbols (Simplified): Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AILewis dot symbols illustrate valence electrons, crucial for understanding chemical bonding. Main group elements (groups 1A to 8A) have valence electrons equal to their group number, while transition metals require consideration of both s and d electrons. The element symbol represents the nucleus and core electrons, while surrounding dots indicate valence electrons. For instance, lithium, sodium, and potassium each have one dot, reflecting their group 1A status. Understanding these concepts is essential for grasping electron configurations and chemical reactivity.
Lewis Dot Symbols represent the outer shell electrons of an element.
Lewis Dot Symbols
Lewis Dot Symbols (Simplified) Concept 1
Video transcript

Lewis Dot Symbols (Simplified) Example 1
Video transcript
Here it states which element will possess the most valence electrons. Now one of them I already gave away. Remember, aluminum is in group 3A, so it has 3 valence electrons. Now if we look, we have sulfur, calcium, hydrogen, and bromine. None of them are transition metals, so we don't have to worry about the number of s or d electrons that they possess. They're all main group elements, so look on your periodic table and see what group they belong to. Sulfur is in group 6A, so it has 6 valence electrons. Calcium is in group 2A, so it has 2 valence electrons. Hydrogen is in group 1A, so it has 1 valence electron. And then finally, bromine is in group 7A, so it has 7 valence electrons. So we'd see that bromine is the one that has the most valence electrons at 7.
Lewis Dot Symbols (Simplified) Concept 2
Video transcript
Now when it comes to drawing a Lewis dot symbol, it's important to understand what is meant by element symbol versus the surrounding dots. When we say element symbol, that equals the nucleus of the element as well as its core electrons. When we say the surrounding dots, these equal the element's valence electrons. Now hydrogen is a little bit weird and so is helium. Both have only one shell, so their valence electrons can also be interpreted as their core electrons, because that's their total number of electrons. Now for the other elements after them though, what we see in red represents the valence electron for that particular element. Now remember, for main group elements, the number of valence electrons V=G, where V is the number of valence electrons and G is the group number. So, you can see that lithium, sodium, and potassium, which are all in group 1a, have one dot. And you can see that the elements in group 6a, oxygen, sulfur, selenium, as well as the ones below them, they all have 6 dots. Now, as always, transition metals are a little bit different. They can have unexpected situations, so we tend to avoid Lewis dot symbols for transition metals. We're going to focus on main group elements. So remember, for a main group element, the number of valence electrons is equal to their group number.
Lewis Dot Symbols (Simplified) Example 2
Video transcript
Here it says to draw the Lewis dot symbol for the following element. Here we're dealing with Tellurium. So let's follow the steps. Step 0 says, first, we need to identify the group number of the main group element. Here, Tellurium itself is in group 6a. Next, we're going to ignore transition metals because their electron arrangement causes unpredictable patterns. So don't worry about the transition metals like I said before. Step 1, you're going to place 1 valence electron at a time on the four sides of the element. Start from the top of the element and move clockwise. So we're going to have tellurium, and it's in group 6a, so that means it has 6 valence electrons. So we're going to start off with 1, 2, 3, 4. Remember, we have to get to 6, so we're still missing 2 more. 5, 6. We followed step 2, where it says continue adding electrons, pairing them up until you reach the appropriate number of valence electrons. Here we need to get to 6 valence electrons, which is what we just did. So this here represents the Lewis dot symbol for tellurium.
Draw the Lewis Dot symbol for the following element:Sr
Draw the Lewis Dot symbol for the following element:Pb
Draw the electron-dot symbol for the following element:Rn
Here’s what students ask on this topic:
What are Lewis dot symbols and what do they represent?
Lewis dot symbols, also known as electron dot diagrams, are diagrams that represent the valence electrons of an atom or ion. The element symbol in the center represents the nucleus and core electrons, while the surrounding dots indicate the valence electrons. These symbols are crucial for understanding chemical bonding and reactivity. For main group elements (groups 1A to 8A), the number of valence electrons is equal to their group number. For example, aluminum in group 3A has three valence electrons. Transition metals are more complex, as their valence electrons include both s and d electrons.
 Created using AI
Created using AIHow do you determine the number of valence electrons for main group elements using Lewis dot symbols?
To determine the number of valence electrons for main group elements using Lewis dot symbols, you look at the element's group number in the periodic table. Main group elements are found in groups 1A to 8A. The group number corresponds to the number of valence electrons. For example, elements in group 1A, like lithium, sodium, and potassium, have one valence electron, so their Lewis dot symbols will have one dot. Similarly, elements in group 6A, like oxygen and sulfur, have six valence electrons, so their Lewis dot symbols will have six dots.
 Created using AI
Created using AIWhy are Lewis dot symbols for transition metals more complex than for main group elements?
Lewis dot symbols for transition metals are more complex because their valence electrons include both s and d electrons. Unlike main group elements, where the number of valence electrons is equal to the group number, transition metals have varying numbers of d electrons that can participate in bonding. This variability makes it difficult to represent their valence electrons with simple dot diagrams. As a result, Lewis dot symbols are typically avoided for transition metals, and more advanced methods are used to describe their electron configurations and bonding behavior.
 Created using AI
Created using AIHow do you draw a Lewis dot symbol for an element like oxygen?
To draw a Lewis dot symbol for an element like oxygen, follow these steps: First, identify the element's group number in the periodic table. Oxygen is in group 6A, so it has six valence electrons. Write the element symbol 'O' to represent the nucleus and core electrons. Then, place six dots around the symbol to represent the valence electrons. Arrange the dots in pairs around the four sides of the symbol, starting with one dot on each side before pairing them up. The final diagram should have two pairs of dots and two single dots around the 'O' symbol.
 Created using AI
Created using AIWhat is the significance of valence electrons in chemical bonding?
Valence electrons are the outermost electrons of an atom and play a crucial role in chemical bonding. They are the electrons involved in forming bonds with other atoms. In covalent bonding, atoms share valence electrons to achieve a stable electron configuration, often resembling the nearest noble gas. In ionic bonding, atoms transfer valence electrons to achieve stability, resulting in positively and negatively charged ions that attract each other. Understanding the number and arrangement of valence electrons helps predict how atoms will interact and bond, which is essential for understanding chemical reactivity and properties.
 Created using AI
Created using AIYour Introduction to Chemistry tutor
- Write the group number and draw the Lewis symbol for each of the following elements: e. gallium
- Write the group number and draw the Lewis symbol for each of the following elements: c. calcium
- An unidentified element is found to have an electron configuration by shell of 2 8 18 8 2. To what group and p...
- Consider the following Lewis symbols for elements X and Y: (6.1, 6.2, 6.5) c. What ions would be formed by X...
- Consider an ion with the symbol X²⁺ formed from a representative element. (6.1, 6.2, 6.3) b. What is the Lewi...
- Consider an ion with the symbol Y³⁻ formed from a representative element. (6.1, 6.2, 6.3) b. What is the Lewi...