In Lewis dot structures, cations have fewer valence electrons because remember, cations are positive, positive because they're losing electrons. Anions have more valence electrons; an anion is a negative ion, negative because it's gaining electrons. So using this, let's answer this example question. It says draw the Lewis dot structure for the following anion, BCl4-1. Alright. So here we have to determine the total number of valence electrons of the structure. And recall that valence electrons equal the group number of the element. So here we have boron which is in group 3A, and there's one of it. We have chlorine which is in group 7A and there's 4 of them. That's 28. And then remember that this minus one means we've gained an electron. So we're going to add 1 electron to this mix, giving us a total of 32 valence electrons. Now we're going to place the least electronegative element in the center, and connect all elements with single bonds. We're going to follow our bonding preferences guide to determine atom connectivity. Now, if you haven't watched my video on bonding preferences, I highly suggest you go back and take a look because it helps you to identify what kind of bonds these elements like to make. 2 bonds, 3 bonds, etcetera. So here we're going to place boron in the center, and we're going to make single bonds to each of the chlorines. Now we're going to add electrons to all surrounding elements until they have 8 electrons, because we're trying to follow the octet rule. Now remember, hydrogen doesn't follow the octet rule; it follows the duet rule. It only wants 2 valence electrons around it, so that it can be just like helium. So I'm adding enough electrons so that each chlorine is following the octet rule. Now place any remaining electrons on the central element or central atom. So we've already used all 32 of our electrons here, so there's none left. So step 5 says that we need to place the ion in brackets and its charge in the top right corner. So that's right. So for ions, once you draw the Lewis dot structure, you have to put it in brackets with the charge on the outside. So brackets around it, and you'd put the minus charge here. Here I'm going to bring it down so we can see it better. So here goes boron with the 4 chlorines that it's attached to. So here goes our 4 chlorines that we're attached to. Since it's an ion, put it in brackets, and the charge on the outside and top right corner. Now for cations, this is not a cation, but for cations removal of valence electrons from the central element. So just keep that in mind when dealing with cations. So these would be the rule that we use in order to draw either cations or anions when asked to determine the Lewis dot structure.
- 1. The Chemical World9m
- 2. Measurement and Problem Solving2h 25m
- 3. Matter and Energy2h 15m
- Classification of Matter18m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Temperature (Simplified)9m
- Law of Conservation of Mass5m
- Nature of Energy5m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Heat Capacity16m
- Thermal Equilibrium (Simplified)8m
- Intensive vs. Extensive Properties13m
- 4. Atoms and Elements2h 33m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Phases (Simplified)8m
- Periodic Table: Main Group Element Charges12m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- 5. Molecules and Compounds1h 50m
- Law of Definite Proportions9m
- Periodic Table: Elemental Forms (Simplified)6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Acids18m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Calculating Molar Mass9m
- 6. Chemical Composition1h 23m
- 7. Chemical Reactions1h 43m
- 8. Quantities in Chemical Reactions1h 16m
- 9. Electrons in Atoms and the Periodic Table2h 32m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)20m
- The Electron Configuration: Condensed4m
- Ions and the Octet Rule9m
- Valence Electrons of Elements (Simplified)5m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)7m
- Electron Arrangements5m
- The Electron Configuration: Exceptions (Simplified)12m
- 10. Chemical Bonding2h 10m
- Lewis Dot Symbols (Simplified)7m
- Ionic Bonding6m
- Covalent Bonds6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Bonding Preferences6m
- Multiple Bonds4m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)7m
- Molecular Geometry (Simplified)9m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)14m
- Molecular Polarity (Simplified)7m
- 11 Gases2h 15m
- 12. Liquids, Solids, and Intermolecular Forces1h 11m
- 13. Solutions3h 1m
- 14. Acids and Bases2h 14m
- 15. Chemical Equilibrium1h 27m
- 16. Oxidation and Reduction1h 33m
- 17. Radioactivity and Nuclear Chemistry53m
Lewis Dot Structures: Ions (Simplified): Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIIn Lewis dot structures, cations have fewer valence electrons due to electron loss, while anions have more from electron gain. For the anion BCl4-, calculate total valence electrons: boron (3) + 4 chlorines (28) + 1 (for the charge) = 32. Place boron at the center, bond it to four chlorines, and ensure each chlorine follows the octet rule. Finally, enclose the structure in brackets with the charge in the top right corner. Understanding these principles is essential for accurately representing ionic compounds.
Lewis Dot Structures of Ions involves losing or gaining valence electrons to draw the most likely structure.
Lewis Dot Structures of Ions
Lewis Dot Structures: Ions (Simplified) Concept 1
Video transcript
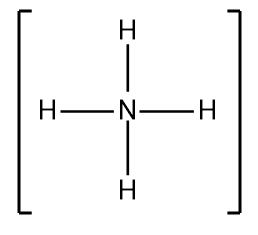
Draw the Lewis Dot Structure for the following cation:NH4+.
Determine the Lewis Dot Structure for the following ion:O22–.
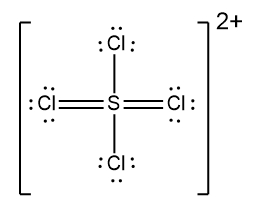
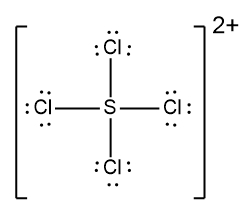
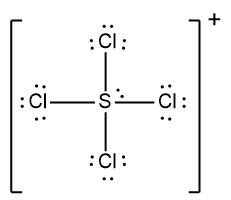
Determine the Lewis Dot Structure for the following ion:SCl42+.
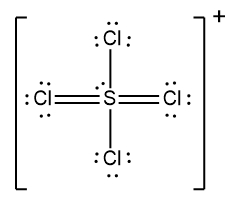
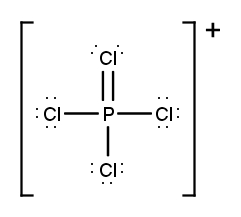
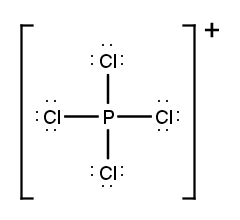
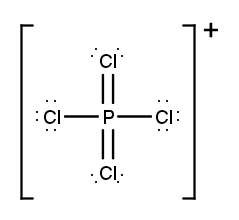
Draw the Lewis Dot Structure for the following ion:PCl4+.
Here’s what students ask on this topic:
How do you draw the Lewis dot structure for anions?
To draw the Lewis dot structure for anions, follow these steps: First, determine the total number of valence electrons by adding the group numbers of each element and accounting for the extra electrons due to the negative charge. For example, in BCl4-, boron (group 3A) has 3 valence electrons, and each chlorine (group 7A) has 7, totaling 28 for four chlorines. Add 1 more electron for the negative charge, giving 32 valence electrons. Place the least electronegative element (boron) in the center and connect it to the chlorines with single bonds. Ensure each chlorine follows the octet rule by adding electrons. Finally, enclose the structure in brackets with the charge in the top right corner.
 Created using AI
Created using AIWhat is the significance of the octet rule in Lewis dot structures?
The octet rule is crucial in Lewis dot structures as it states that atoms tend to form bonds until they are surrounded by eight valence electrons, achieving a stable electron configuration similar to noble gases. This rule helps predict the bonding behavior of atoms in molecules and ions. For example, in the anion BCl4-, each chlorine atom must have eight electrons around it, including shared electrons from bonds, to satisfy the octet rule. This ensures the structure is stable and accurately represents the molecule or ion.
 Created using AI
Created using AIWhy do cations have fewer valence electrons in Lewis dot structures?
Cations have fewer valence electrons in Lewis dot structures because they lose electrons to achieve a positive charge. When an atom loses one or more electrons, it becomes a cation with a positive charge. For example, if a sodium atom (Na) loses one electron, it becomes Na+ with no valence electrons in its outer shell. This loss of electrons is reflected in the Lewis dot structure, where fewer dots (representing valence electrons) are shown around the cation.
 Created using AI
Created using AIHow do you determine the total number of valence electrons in a polyatomic ion?
To determine the total number of valence electrons in a polyatomic ion, sum the valence electrons of each atom in the ion and adjust for the ion's charge. For example, in BCl4-, boron (group 3A) has 3 valence electrons, and each chlorine (group 7A) has 7, totaling 28 for four chlorines. Since the ion has a -1 charge, add one more electron, resulting in a total of 32 valence electrons. This total is used to draw the Lewis dot structure.
 Created using AI
Created using AIWhat are the steps to draw the Lewis dot structure for BCl4-?
To draw the Lewis dot structure for BCl4-, follow these steps: 1) Calculate the total valence electrons: Boron (3) + 4 Chlorines (28) + 1 (for the charge) = 32. 2) Place the least electronegative element (boron) in the center. 3) Connect boron to each chlorine with single bonds. 4) Add electrons to each chlorine to satisfy the octet rule. 5) Place any remaining electrons on the central atom. 6) Enclose the structure in brackets with the charge in the top right corner.
 Created using AI
Created using AI