Here are the essential concepts you must grasp in order to answer the question correctly.
Activation Energy
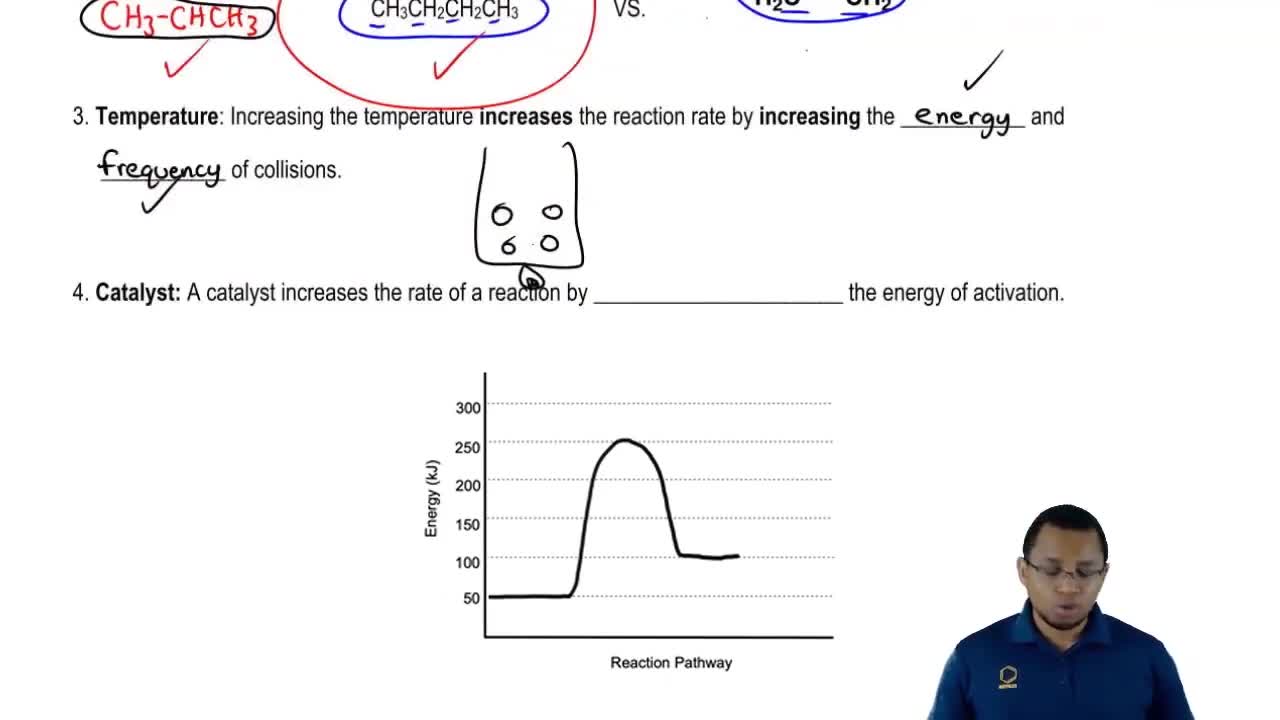
Activation energy is the minimum energy required for a chemical reaction to occur. It represents the energy barrier that reactants must overcome to transform into products. A lower activation energy means that more molecules have sufficient energy to react, thus increasing the reaction rate.
Recommended video:
Energy Diagrams Concept 2
Catalysts
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. They work by providing an alternative reaction pathway with a lower activation energy. Importantly, catalysts affect both the forward and reverse reactions equally, thereby influencing the overall reaction dynamics.
Recommended video:
Rate of Reaction Concept 7
Equilibrium and Reaction Rates
In a reversible reaction, the rates of the forward and reverse reactions are equal at equilibrium. The activation energy for the reverse reaction can be determined by the difference in energy between the reactants and products. If a catalyst lowers the activation energy for the forward reaction, it will similarly lower the activation energy for the reverse reaction, maintaining the balance of reaction rates.
Recommended video:
Rate of Reaction Concept 1