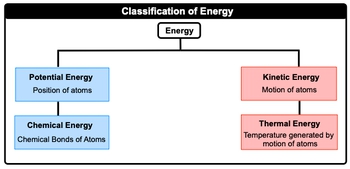
Thermochemistry is the study of matter and energy associated with chemical reactions or physical changes. Energy itself is just the capacity to do work or to produce heat. Now when we talk about energy, realize that there are different types of energy, but in this chapter, we're only going to focus on a selected few. Now when we take a look at the big picture, energy itself, we can say here that it can be broken down initially into either the position of atoms or the motion of atoms. When we're talking about the position of atoms, this is just simply our potential energy, our energy of position. And then if we're talking about the movement or motion of atoms, this is connected to kinetic energy. Now both potential energy and kinetic energy can be further broken down into other types of energy. Potential energy, we can connect it to another one which deals with the chemical bonds of atoms. This is just simply chemical energy. And then kinetic energy can be broken down further into energy associated with temperature generated by motion of atoms. So this would be called thermal energy. So just remember, energy is just the capacity to work and to produce heat. And when we're talking about energy in this chapter, we're mainly concerned with these different types of energy forms. Now, of course, there are other types of energy that exist. We'll go into those in later chapters, but for now just remember these particular four.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Nature of Energy: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIThermochemistry examines the relationship between matter and energy during chemical reactions and physical changes. Energy, defined as the capacity to do work or produce heat, can be categorized into potential energy, related to atomic position, and kinetic energy, associated with atomic motion. Key forms include chemical energy (a type of potential energy) and thermal energy (a type of kinetic energy). The SI unit for energy is the Joule, with conversion factors such as 1 calorie = 4.184 joules and 1 kilowatt hour = 3.6 × 106 joules.
Thermochemistry is the study of matter and energy associated with chemical reactions or physical changes.
Classification of Energy
Nature of Energy
Video transcript
Nature of Energy
Video transcript
In our discussion of the concept of energy, it's important to remember that the SI unit for energy is Joule, and it's named after the English scientist James Joule. When it comes to joules, we're going to say that there are 3 particular types of conversion factors associated with it.
We're going to say here for the first one, we have one calorie which is lowercase c, this equals 4.184 joules. Next, we have 1 capital C calorie, this one is associated with food nutrition. This particular calorie equals 4184 joules. And then finally, kilowatt-hours. Usually, when we talk about an electrical bill, it's associated with kilowatt-hours. So we're going to say here kilowatt-hours equals 3.6×106 joules.
These are 3 conversion factors associated with our SI unit for energy. Also realize here that I didn't put purple boxes around them, so usually, you're not expected to memorize them. They're often given to you within the question or they're given to you on a formula sheet when taking an exam. But here, just again remember, these are 3 common types of conversion factors associated with joules.

Nature of Energy Example 1
Video transcript
Here it states, which of the following statements deals with potential energy without non-chemical energy associated with it. Remember, potential energy is just the energy of position, whereas chemical energy, which is an offshoot of potential energy, is just associated with the chemical bonds of atoms. Alright. So if we take a look here, it says a car traveling with a velocity of 51 meters per second with a mass of 1,250 kilograms. Here we're talking about velocity, we're talking about the motion of this car, so this is more associated with kinetic energy. So, this is out.
Next, your chemistry book sitting on a table counter near the trash can, weighing 12 newtons at a height of 1.2 meters. So make sure it doesn't fall into the trash can. Here, they're just talking about the position of the chemistry book. It's at a certain height, and we're talking about a force on it. Here, this is purely potential energy, so this would be our answer. But let's look at the other options.
A chunk of coal being thrown into a furnace to generate heat. Here we're talking about heat being generated from this. This is closely related to thermal energy. And then next, we have the warmth coming from a campfire. So again, we're talking about temperature, we're talking about heat. So this is a version of kinetic energy in the form of thermal energy.
So, out of all the choices, the only one that is purely potential energy without talking about chemical energy would have to be option B.
An energy efficient refrigerator uses 780 kWh of electrical energy per year. How many kilocalories of electricity does it use in three years?
2.0 x 106 kcal
8.3 x 105 kcal
2 x 106 kcal
8.3 x 106 kcal
Do you want more practice?
Here’s what students ask on this topic:
What is thermochemistry and why is it important?
Thermochemistry is the study of the relationship between matter and energy during chemical reactions and physical changes. It is important because it helps us understand how energy is transferred and transformed in chemical processes, which is crucial for various applications such as energy production, material synthesis, and environmental science. By studying thermochemistry, we can predict the energy changes that occur in reactions, optimize industrial processes, and develop new technologies for energy efficiency.
 Created using AI
Created using AIWhat are the different types of energy discussed in thermochemistry?
In thermochemistry, energy is categorized into two main types: potential energy and kinetic energy. Potential energy is related to the position of atoms and includes chemical energy, which is stored in chemical bonds. Kinetic energy is associated with the motion of atoms and includes thermal energy, which is generated by the movement of atoms. These forms of energy are crucial for understanding how energy is stored and transferred in chemical reactions and physical changes.
 Created using AI
Created using AIWhat is the SI unit for energy and what are some common conversion factors?
The SI unit for energy is the Joule (J), named after the English scientist James Joule. Common conversion factors include: 1 calorie (lowercase 'c') = 4.184 joules, 1 Calorie (uppercase 'C', used in food nutrition) = 4184 joules, and 1 kilowatt hour (kWh) = 3.6 × 106 joules. These conversion factors are often provided in questions or formula sheets during exams, so memorization is not always necessary.
 Created using AI
Created using AIHow is potential energy different from kinetic energy in the context of thermochemistry?
In thermochemistry, potential energy is the energy related to the position of atoms, such as the energy stored in chemical bonds (chemical energy). Kinetic energy, on the other hand, is the energy associated with the motion of atoms, such as the energy generated by their movement (thermal energy). Understanding the difference between these two types of energy is essential for analyzing how energy is stored and transferred during chemical reactions and physical changes.
 Created using AI
Created using AIWhy is it important to understand energy conversion factors in thermochemistry?
Understanding energy conversion factors in thermochemistry is important because it allows us to accurately measure and compare energy changes in different units. For example, knowing that 1 calorie = 4.184 joules helps us convert energy values from one unit to another, which is essential for calculations in experiments, industrial processes, and everyday applications like understanding nutritional information or electricity usage. Accurate energy conversions ensure that we can effectively analyze and optimize energy-related processes.
 Created using AI
Created using AIYour GOB Chemistry tutor
- Discuss the changes in the potential and kinetic energy of a roller-coaster ride as the roller-coaster car cli...
- Indicate whether each of the following statements describes potential or kinetic energy: c. the energy in a l...
- Indicate whether each of the following statements describes potential or kinetic energy: b. kicking a ball
- Using the energy values for foods (see TABLE 3.7), determine each of the following (round off the answer for e...
- Using the energy values for foods (see TABLE 3.7), determine each of the following (round off the answer for e...
- For dinner, Charles had one cup of clam chowder, which contains 16 g of carbohydrate, 12 g of fat, and 9 g of ...
- A patient receives 3.2 L of intravenous (IV) glucose solution. If 100. mL of the solution contains 5.0 g of gl...
- What is a catalyst, and what effect does it have on the activation energy of a reaction?
- If a catalyst changes the activation energy of a forward reaction from 28.0 kcal/mol to 23.0 kcal/mol, what ef...
- Using energy values from TABLE 3.8, determine each of the following: d. If expending 3500 kcal is equal to a ...
- Using energy values from TABLE 3.8, determine each of the following: c. If Charles consumes 1800 kcal per day...
- b. For the amount of exercise that Charles did for one week in part a, if expending 3500 kcal is equal to a lo...
- For the unbalanced combustion reaction shown, 1 mol of ethanol, C2H5OH, releases 327 kcal (1370 kJ): C2H5OH ...
- A 70.0-kg person had a quarter-pound cheeseburger, french fries, and a chocolate shake. (3.5) d. Using TABL...
- The relationship between the nutritional unit for energy and the metric unit is 1 calorie = 1 kcal. a. One don...
- When a 0.66-g sample of olive oil is burned in a calorimeter, the heat released increases the temperature of 3...
- When 1.0 g of gasoline burns, it releases 11 kcal. The density of gasoline is 0.74 g/mL. (3.4, 3.6) b. If a t...
- A patient is receiving 3000 mL/day of a solution that contains 5 g of dextrose (glucose) per 100 mL of solutio...
