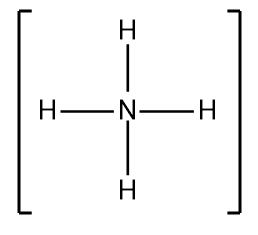
In understanding Lewis dot structures, it's essential to recognize the behavior of cations and anions regarding their valence electrons. Cations, which are positively charged ions, have fewer valence electrons due to the loss of electrons, while anions, negatively charged ions, have more valence electrons because they gain electrons. This foundational knowledge is crucial when constructing Lewis dot structures.
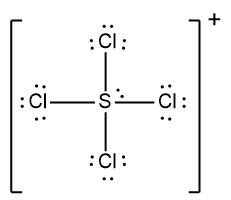
For example, to draw the Lewis dot structure for the anion BCl4-, we first need to calculate the total number of valence electrons. The number of valence electrons corresponds to the group number of the elements in the periodic table. Boron (B) is in group 3A, contributing 3 valence electrons, and chlorine (Cl) is in group 7A, contributing 7 valence electrons. Since there are four chlorine atoms, we calculate:
Valence electrons from B: 3
Valence electrons from Cl: 4 × 7 = 28
Total from B and Cl: 3 + 28 = 31
Adding one more electron due to the negative charge: 31 + 1 = 32 valence electrons.
Next, we place the least electronegative element, boron, at the center and connect it to the four chlorine atoms with single bonds. Following the bonding preferences, we ensure that each chlorine atom achieves an octet by adding electrons around them until they each have 8 electrons. It's important to remember that hydrogen follows the duet rule, desiring only 2 electrons, but in this case, we are only dealing with chlorine atoms.
After distributing the 32 electrons, we find that all are used up, with each chlorine atom surrounded by 8 electrons. Since there are no remaining electrons, we then enclose the entire structure in brackets to indicate that it is an ion, placing the charge in the top right corner. Thus, the final representation of the Lewis dot structure for BCl4- shows boron at the center with four chlorine atoms bonded to it, all enclosed in brackets with the negative charge indicated.
In summary, when constructing Lewis dot structures for ions, it is vital to account for the total number of valence electrons, correctly position the central atom, ensure surrounding atoms satisfy their electron requirements, and properly denote the ionic charge in the final structure.