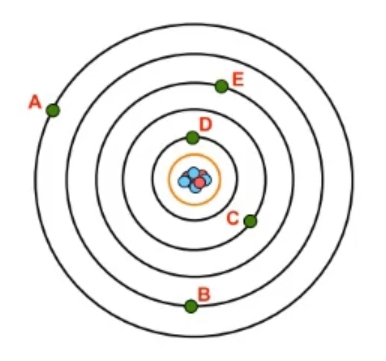
Now recall a shell, which uses the variable n, is a grouping of electrons surrounding the nucleus that ties into their potential energy, so their energy of position. Now we're going to say as the value of n increases, then both the size and energy level of an atomic orbital will increase. And we're going to say here that the energy levels, also the shell numbers of an atom, can be tied to the periods or rows of the periodic table. So, here we have an atom. This atom has its nucleus, and we can see that I have given it 7 shells. Right? Remember, each black orbital represents a shell; 1st shell all the way to the 7th shell here.
We'll realize here that the shells of an atom are directly related to the periods or rows of the periodic table. So, remember, your periods or rows of the periodic table go from left to right. So this is row 1, so this is shell 1, shell 2, 3, 4, 5, 6, and 7. Now currently, there are 7 rows in the periodic table, but what you need to understand about the periodic table is that it is dynamic. A lot of these elements found in the 7th row of the periodic table were discovered within the past decade. A bunch of these here were formally named in the past 10 years. So theoretically, as we move to new planets, as we explore more of Earth, as our technology gets more advanced, we're going to create and find new elements.
So eventually, there's going to be an 8th row of elements, and then a 9th row, and so on and so forth. We're only limited by our imagination and our ingenuity. The number of elements can continuously increase again as we discover them and as we create them. Now we're going to say because of this, the limitation of the n value is that it must be an integer, remember, a whole number, from 1, because the smallest number shell can be is 1, to infinity. There's an infinite number of possible shells. Again, we're only limited by our resources, our imagination, and our ingenuity. We can create new elements with 10 shells, 12 shells, and so on.
So keep in mind the relationship between rows of the periodic table and shell numbers of an atom and realize the limitation of it is that it can be any number from 1 to infinity.