Guys, in this new video, we're going to take a look at gamma emissions. So here we're going to say gamma radiation is related to the electromagnetic spectrum. Now, we're going to say gamma rays have the highest energy and therefore they have the lowest or shortest wavelength, and they have the highest frequency. So remember, you have to remember from electromagnetic spectrum theory, that when it comes to energy, energy and frequency are directly proportional. All that means is if one is high, the other one is high. But when it comes to wavelength, wavelength is inversely proportional to both of them. All that means is that wavelength is the opposite of the other two. If they're high, it's low. If it's high, they're low. So it's a complete opposite of frequency and energy, and remember, wavelength is just the distance from one wave to another wave. Where frequency is how many waves you get within one second. So if your distance between waves, the crests, the tops of them, is very large, that means you don't get many waves in a second. But if the wavelength is very small, if the distance between them is very small, you can cram a bunch of them in within one second. Okay. So then you would say the frequency is extremely high and the energy is high. Gamma rays have the highest behind cosmic rays. Cosmic rays would actually be a little bit higher than gamma rays. We usually don't hear about this in lecture, but in lecture, so strictly lecture, we're going to say gamma rays have the highest energy, and therefore they have the highest frequency, and therefore the lowest or shortest wavelength. If you went beyond just general chemistry, you go into physics and other, higher-level sciences, they'd start talking about cosmic rays, which are then even higher than gamma rays. But for right now, just focused on the simple electromagnetic spectrum, gamma rays are going to have the highest energy.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Gamma Emission: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIGamma radiation is a form of electromagnetic radiation with the highest energy, shortest wavelength, and highest frequency. It is represented by the symbol . Gamma emissions occur when an electron absorbs energy, causing it to jump to a higher energy shell or orbital. Despite having the lowest ionizing power, gamma radiation is extremely penetrating and toxic to biological tissues, making exposure highly dangerous.
The gamma particle does not create a new element like the other radioactive particles, but instead causes the excitation of electrons within an element.
Understanding Gamma Emission
A gamma particle has no atomic mass and no atomic number and is represented by the sign gamma.

Gamma Emission Concept 1
Video transcript
Gamma radiation is involved in the electromagnetic spectrum. Gamma rays possess the highest energy, while radio waves have lowest energy in terms of the spectrum.
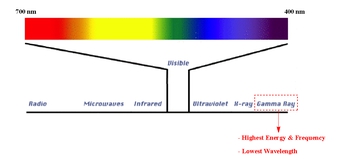
Gamma Emission Concept 2
Video transcript
Now we're going to say a gamma particle can be represented by 00 and the gamma symbol is this. Now, you're going to say because it's 00, you should realize that a gamma ray actually does not cause any change in your atomic mass or atomic number. And because of that, we usually see it happening with alpha decay or beta decay. But what's the whole purpose of gamma emission then? Well, we're going to say when it comes to gamma emission, it has to do with the absorption of energy. So here we're going to say this wavy live line represents energy, and this electron is in our first shell in our atom. So here in absorption, the electron is going to absorb that excess energy and become excited, and use that extra energy just absorbed to jump up to either a higher shell number or to a higher orbital number. So basically, if you go from 1s to 2s. So you're going from the first shell to the second shell, that represents absorption. You could also go from 3s to 3d. You can skip 3p altogether, and just jump up straight to 3d. Both of these examples represent absorption. The first one represents absorption where you jump from one shell to a higher shell, and then the 3s to 3d represents you absorbing energy and jumping up from a lower orbital to a higher orbital within the same shell. Both of them begin with the number 3, so they're both within the third shell of your atom, but d orbitals have more energy than s orbitals. So if we solve this, we'd have 4020 calcium. It undergoes a gamma emission, and we'd say that that calcium has an electron that had just absorbed energy and it's going to become excited. So we put a little asterisk by it to show that it's in an excited state. That will represent a gamma emission. Now, we're going to say that gamma particles, they have the lowest ionizing power, but they have the highest penetrating power. So if you're ever exposed to gamma emission, like gamma radiation, it's basically a done deal. You're not going to survive. Gamma radiation is extremely toxic to living tissue and biological systems. So any exposure to even the smallest amount of gamma radiation would completely eviscerate all the living cells and tissues within your body. It has the lowest ionizing power, but it's still extremely dangerous.

Gamma Particles have lowest ionizing power, but are the most dangerous because of their highest penetrating power.

Gamma Emission Example 1
Video transcript
Now, if we go to this example, it says which of the following represents an element that has experienced a gamma emission? Here we have electron configurations for elements. Now remember, the normal pattern is, you go from 1s to 2s to 2p to 3s to 3p, 4s, etcetera. And remember, gamma emission involves the excitation or the absorption of energy gets you into an excited state, so you could actually have an electron skipping an entire orbital, like it's supposed to be in the 2s, but it gains so much energy it skips 2s and goes to 2p. Or you could have an electron gaining even more energy and going from 2s to 3s. So, if we look, the one that fits this excited state or this gamma emission state would have to be sodium, because we're supposed to see 1s followed by 2s, followed by 2p, followed by 3s. But what must have happened? This electron in here must have absorbed energy and therefore able to jump to a higher energy state. So the next one would be 3p. So it skipped the 3s and went straight to 3p. So this represents an element that has gained sufficient energy to become excited, which represents a gamma emission. So remember, gamma emission may not change your atomic mass or atomic number like alpha decay or beta decay, but it still does affect the atom in some way.
Do you want more practice?
Here’s what students ask on this topic:
What is gamma radiation and how does it relate to the electromagnetic spectrum?
Gamma radiation is a form of electromagnetic radiation characterized by the highest energy, shortest wavelength, and highest frequency within the electromagnetic spectrum. It is situated beyond X-rays on the spectrum. The relationship between energy, frequency, and wavelength is crucial: energy and frequency are directly proportional, meaning if one is high, the other is also high. Conversely, wavelength is inversely proportional to both energy and frequency, so a high energy and frequency correspond to a short wavelength. Gamma rays are highly penetrating and can be extremely dangerous to biological tissues.
 Created using AI
Created using AIWhat happens during gamma emission in terms of atomic structure?
During gamma emission, an electron within an atom absorbs energy, causing it to become excited and jump to a higher energy shell or orbital. This process does not change the atomic mass or atomic number of the element, as gamma rays are represented by 0/0. For example, if a calcium atom (40/20 Ca) undergoes gamma emission, an electron absorbs energy and moves to a higher energy state, indicated by an asterisk to show its excited state. This emission is highly penetrating but has low ionizing power.
 Created using AI
Created using AIWhy is gamma radiation considered highly dangerous to biological tissues?
Gamma radiation is considered highly dangerous to biological tissues due to its extremely high penetrating power. Even though it has the lowest ionizing power among radiation types, its ability to penetrate deeply into tissues makes it highly toxic. Exposure to gamma radiation can eviscerate living cells and tissues, leading to severe biological damage or death. This makes even minimal exposure to gamma radiation potentially lethal.
 Created using AI
Created using AIHow does gamma radiation compare to cosmic rays in terms of energy?
Gamma radiation has the highest energy within the electromagnetic spectrum, just below cosmic rays. Cosmic rays possess even higher energy levels than gamma rays, but they are less commonly discussed in general chemistry and more often in advanced physics. For the purposes of basic electromagnetic spectrum studies, gamma rays are considered to have the highest energy, frequency, and shortest wavelength.
 Created using AI
Created using AIWhat is the significance of the 0/0 symbol in gamma radiation?
The 0/0 symbol in gamma radiation indicates that gamma rays do not cause any change in the atomic mass or atomic number of an element. This is because gamma emission involves the release of energy without altering the number of protons or neutrons in the nucleus. The symbol helps to differentiate gamma radiation from other types of radioactive decay, such as alpha or beta decay, which do change the atomic structure.
 Created using AI
Created using AIYour GOB Chemistry tutor
- Bone and bony structures contain calcium and phosphorus. b. During nuclear tests, scientists were concerned t...
- a. Technetium-99m emits only gamma radiation. Why would this type of radiation be used in diagnostic imaging r...
- Write the balanced nuclear equation for each of the following: (5.1, 5.2) e. In-113m (γ emission)
- Complete each of the following nuclear equations: (5.2) d. ²³ᵐ₁₂Mg → ? + ⁰₀γ
