Hey, everyone. So when it comes to nitrogenous bases, we're going to say there are 5 different nitrogenous bases that are grouped into 2 categories. We're going to have our pyrimidines versus our purines. Now, pyrimidines, these are our single-ringed molecules, and our purines are our double-ringed molecules. Now, here we have memory tools to help us remember which is which. When it comes to our Pyrimidines, we're going to have our Cytosine, our Thymine, and our Uracil. When it comes to Thymine, this is only found within DNA and Uracil is only found within RNA. Later on, we'll talk about their given structures. Right now, we're only caring about grouping them. So these 3 nitrogen bases all are single, molecules, single-ringed molecules. And our memory tool here is that creepy tombs under pyramids. Pyramids, remidines, creepy for cytosine, tombs for thymine, and under for uracil. Here, their one-letter abbreviations would be c, t, u. On the other side, we have our purines, which are our adenine and guanine, so a and g. So it's 2 rings fused together when it comes to these structures, and our memory tool here is pure as gold. Pure for purines, s for adenine, and then g gold guanine. Alright. So just remember, we have our creepy tombs under pyramids and pure as gold to help us group our 5 nitrogenous bases into their 2 categories, being either single-ringed molecules or double-ringed molecules.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Nitrogenous Bases: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AINitrogenous bases are categorized into pyrimidines and purines. Pyrimidines, which include cytosine (C), thymine (T), and uracil (U), are single-ringed structures. Purines, adenine (A) and guanine (G), feature double-ringed structures. Understanding their structures is crucial: uracil has two carbonyl groups, thymine adds a methyl group, and cytosine contains an amine group. Purines consist of two fused rings with four nitrogen atoms. These foundational concepts are essential for grasping nucleic acid structure and function in biological systems.
Nitrogenous Bases Concept 1
Video transcript
Nitrogenous Bases Example 1
Video transcript
So in this example, it says label each nitrogen space as a pyrimidine (PY) or a purine (PU). So remember when it comes to our pyrimidines, we're going to say here creepy tombs under pyramids. So here, if these represent our pyrimidines. When we say creepy here, creepy here stands for cytosine. So this will be PY. Tombs is for thymine. So PY. Under here is uracil. So PY. And then for purines, we're going to say purines are pure as gold. So our purines pure. As is for adenine. So here this would be PU. And then, gold here is for guanine. So this is how we label each of the following nitrogenous bases based on these two memory tools. We can group them into pyrimidines or purines.
The four nitrogenous bases commonly found in DNA are:
Uracil, cytosine, guanine, thymine.
Adenine, thymine, cytosine, uracil.
Uracil, adenine, cytosine, guanine.
Adenine, thymine, cytosine, guanine.
None are correct.
Nitrogenous Bases Concept 2
Video transcript
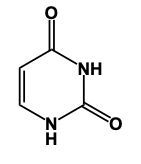
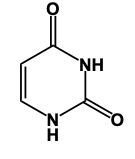
In this video, we'll learn some tricks to help us remember the structures of different pyrimidines. Here, first of all, when we say pyrimidine, this is the general structure of pyrimidine. The 3 pyrimidines that we have are just modifications of this original structure, and it all begins with Uracil. Uracil has its 2 nitrogens just like pyrimidine does, but it also has 2 carbonyl groups. So we have a double bond O here and a double bond O here. Now, here, we had a double bond, but we can no longer have a double bond here because then this carbon would be making 5 bonds, which is not allowed. Nitrogen ideally wants to make 3 bonds. To do that, it would have to be connected to a hydrogen. So it would have a connection to the 2 carbons and then the 3rd bond would be to the hydrogen. We encounter the same issue here with this bottom nitrogen. We can't have a double bond like we have here because then this carbonyl carbon would be making 5 bonds; carbon can only have up to 4. So, for nitrogen to reach its ideal number of 3 bonds, it would be connected to a hydrogen. This represents the structure of uracil. Remember, we have our 2 carbonyls here and then each of the nitrogens to make their 3rd bond connects to a hydrogen. Now, here, Uracil, the other two pyrimidines are just modifications of this: thymine and cytosine.
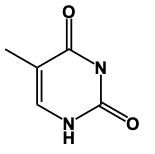
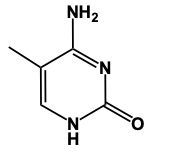
Thymine is very similar in structure to uracil; the only difference is the presence of a methyl group. The methyl group would be located here, and we'd still have our 2 carbonyls here and here, and our nitrogens would still have a hydrogen attached. This is thymine.
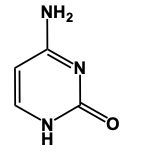
Cytosine is a little trickier, but just remember, we substitute a cyamine group. So, understanding the structure of cytosine: we would still have our carbonyl here and a hydrogen on this nitrogen here. But now, we retain this double bond just like this nitrogen here possesses a double bond, achieving its 3 bonds, so it does not need an additional hydrogen. Noting the cyamine, "amine" group pronounces the same as an amine, which is a -NH2 group. So rather than having 2 carbonyls, we have 1 carbonyl and 1 NH2 group here. This represents our cytosine. Remember, this is the starting structure of pyrimidine. The 3 pyrimidines are merely modifications of it. It all starts with uracil, and from there, you can adapt it to create thymine or cytosine. This is the key to remembering the structures of these different types of nitrogenous bases.
Nitrogenous Bases Example 2
Video transcript
Here in this example question it says, "Complete a structure of Thymine Base." So remember to draw Thymine we need to remember the structure for Uracil. If we can remember the structure for uracil, we just adapt it to give us Thymine. Now, first of all, remember that our pyrimidines have this basic structure involved. We have our nitrogens here. This will make a double bond, double bond here, and double bond here. This is the general structure of a pyrimidine. Uracil, all we do now is we adapt this structure, we'd have 2 carbonyl groups here and here. We'd still have our double bond here. The nitrogens still need to make 3 bonds and they do that by adding an H to each one. This would be Uracil. Thymine, we can get Thymine. Just remember, methyl because it's connected to the Thymine. So here with that size means we have this similar structure. The difference now is we're going to add a methyl group. So we'd still have a double bond here. We'd have our 2 carbonyls still. Each nitrogen would still have an H. Methyl for thymine. The methyl will come off of this carbon here. So this will represent the structure for Thymine. Remember, we were able to do it by first remembering what a pyrimidine looks like in terms of this structure, then remembering modifying this structure to uracil. And then just remember, if you know uracil, Thymine is almost the same except we have a methyl involved. So add the methyl group to the appropriate carbonyl. And there you have it. Thymine represents what we have within the box.
Nitrogenous Bases Concept 3
Video transcript
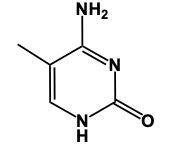
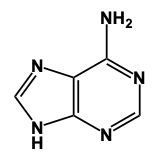
In this video, we'll talk about some tricks that we can remember in order to draw Purine structures. Now, a Purine, the base form is this. We have 2 rings fused together, and we see that we have 4 nitrogens embedded within those rings. Now Adenine. Remember the structure of Adenine, just remember Adenine, adamantine, and Amine is an NH2 group. Here we're just gonna add an NH2 group to this structure. So here we'd still have a double bond on this nitrogen and we would add our amine to this carbon right here, our NH2 group. And this will represent adenine.
Now, guanine, if you remember the structure of guanine, we're gonna say go first. This red oxygen indicates that we have a carbonyl. And there goes our red oxygen. Now because we have that carbonyl carbon there, it cannot make a double bond. Otherwise, it'd be making 5 bonds, right? So a double bond cannot go here. That means this nitrogen is only making 2 bonds. Ideally, it wants to make 3. To get that 3rd bond, it has to connect to a hydrogen. So guanine, we have the go part. And then the second part is amine. So again, we have amine involved. An amine is an NH2 group. That NH2 group would go right here. So this represents our structures of Adenine and Guanine. But again, it all originates from the base form of Purine, which is our 2 fused rings with 4 nitrogen atoms embedded within them.
Nitrogenous Bases Example 3
Video transcript
Here it says, complete a structure of the guanine base. So remember, guanine, which is a purine, and its base form of purine would be these 2 nitrogens having double bonds. But we're going to adapt this base form of purine to give us guanine at the end. Now, to remember the structure of guanine, we just have to remember, Go Amine. So the "go," that red oxygen indicates that we have a carbonyl group. We have to get rid of that double bond there because if we didn't, that carbonyl carbon would be making 5 bonds. Carbon can only make up to 4 bonds. Now this gives us an issue though. This nitrogen now isn't making 3 bonds like it ideally wants to; it's only making 2. So in order to make that 3rd bond, it would have to gain an H. Next, we have Amine. Remember, an amine is an NH2 group. That means we'd have to add an NH2 group somewhere to the structure, which would be right here. So here, this will represent the structure of our guanine nitrogenous base. Remember, we've just adapted the base form of our purine molecule in order to get this particular nitrogenous base.
Select a correct structure for U.
Draw a structure for cytosine.
Do you want more practice?
Here’s what students ask on this topic:
What are the differences between pyrimidines and purines?
Pyrimidines and purines are two categories of nitrogenous bases. Pyrimidines, which include cytosine (C), thymine (T), and uracil (U), are single-ringed structures. Thymine is found only in DNA, while uracil is found only in RNA. Purines, on the other hand, include adenine (A) and guanine (G) and feature double-ringed structures. The mnemonic 'creepy tombs under pyramids' helps remember pyrimidines (C, T, U), and 'pure as gold' helps remember purines (A, G). Understanding these differences is crucial for studying nucleic acid structure and function in biological systems.
 Created using AI
Created using AIHow can you remember the structures of pyrimidines?
To remember the structures of pyrimidines, start with the basic pyrimidine structure, which has two nitrogen atoms. Uracil has two carbonyl groups. Thymine is similar to uracil but adds a methyl group. Cytosine has one carbonyl group and an amine group (NH2). Mnemonics can help: 'Thy-methyl' for thymine (indicating the methyl group) and 'Cy-amine' for cytosine (indicating the amine group). These tricks can help you recall the specific modifications that differentiate these bases.
 Created using AI
Created using AIWhat is the structure of adenine and how can you remember it?
Adenine is a purine with a double-ring structure and four nitrogen atoms. To remember adenine's structure, use the mnemonic 'adamine,' which hints at the presence of an amine group (NH2). In adenine, this NH2 group is attached to one of the carbons in the ring structure. This simple mnemonic can help you recall the key feature of adenine's structure.
 Created using AI
Created using AIWhat is the difference between thymine and uracil?
Thymine and uracil are both pyrimidines, but thymine is found only in DNA, while uracil is found only in RNA. Structurally, thymine has a methyl group attached to its ring, whereas uracil does not. This methyl group is the primary difference between the two bases. Remembering 'Thy-methyl' can help you recall that thymine has the additional methyl group compared to uracil.
 Created using AI
Created using AIHow do the structures of purines differ from pyrimidines?
Purines and pyrimidines differ primarily in their ring structures. Purines, which include adenine (A) and guanine (G), have a double-ring structure with four nitrogen atoms. Pyrimidines, which include cytosine (C), thymine (T), and uracil (U), have a single-ring structure with two nitrogen atoms. This structural difference is crucial for the pairing of bases in DNA and RNA, where purines pair with pyrimidines to maintain the double helix structure.
 Created using AI
Created using AIYour GOB Chemistry tutor
- a. What are the four major heterocyclic bases in DNA?
- What are the two structural types of bases in DNA and RNA? Which bases correspond to each type?
- Identify each of the following bases as a purine or a pyrimidine:a. guanine
- Identify each of the following bases as a pyrimidine or a purine: (17.1)a. cytosine
- State whether each of the following components is present in DNA only, RNA only, or both DNA and RNA:c. uracil
- Identify each of the following as a purine or a pyrimidine and name them.b. <IMAGE>