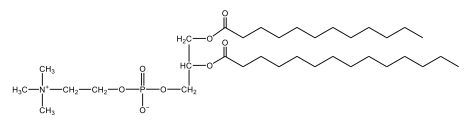
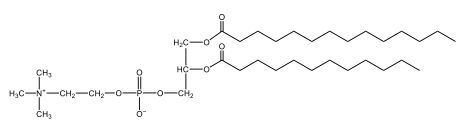
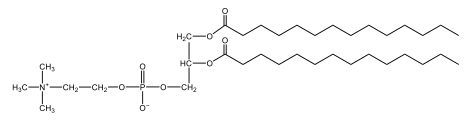
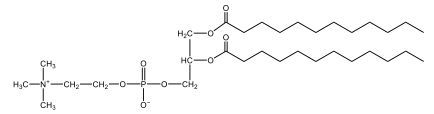
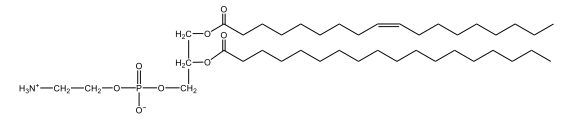
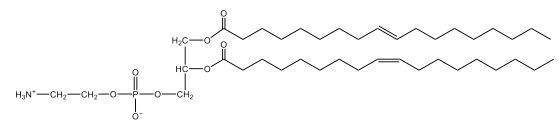
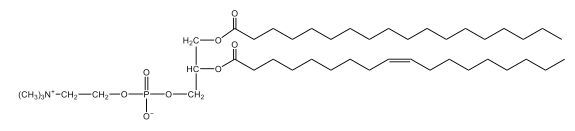
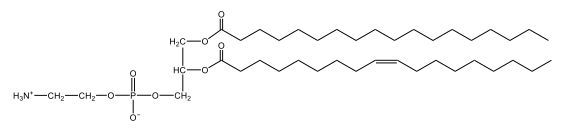
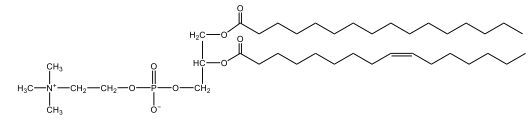
Now, here we're going to say that phospholipids are lipids that contain a phosphate group attached to a glycerol or sphingosine backbone. Here we're going to say, like fatty acids, phospholipids are amphipathic, meaning they have a hydrophilic head and a hydrophobic tail. If we take a look here, we have our illustration of our hydrophilic head. Because it's hydrophilic, it would be a polar head. And then we have our hydrophobic tail, which will mean it's non-polar. Now remember, lipids can be broken down first into fatty acids and steroids. Here we're focusing on the fatty acid portion, which can be further broken down into our waxes, our glycerol lipids, our sphingolipids, and then here we'll talk about our eicosanoids later on. And here, when we're talking about our phospholipids, it's actually shared by these two subclassifications here. Because both of them contain a phosphate group attached to a glycerol or a sphingosine backbone. Now, if we understand that our head is polar and hydrophilic, and our tail is hydrophobic, and this was to be submerged in an aqueous environment, it would orient itself to create this lipid bilayer, where the polar heads are on the exterior and the hydrophobic tails are in the interior. Now, this is important because its ability to do this means that phospholipids are a major component of all cell membranes. Right. So that's an important thing we need to take into account when dealing with phospholipids within aqueous or polar environments. Right? So just keep this in mind in terms of the classifications of phospholipids.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Glycerophospholipids: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIPhospholipids, essential components of cell membranes, consist of a hydrophilic head and a hydrophobic tail, forming a lipid bilayer in aqueous environments. Glycerophospholipids, or phosphoglycerides, have a glycerol backbone with two fatty acids and are classified by their head groups, such as ethanolamine in cefalin and choline in lecithin. These lipids are crucial for cellular structure and function, highlighting the importance of understanding their classifications and properties in biological systems.
Glycerophospholipids Concept 1
Video transcript
Glycerophospholipids Example 1
Video transcript
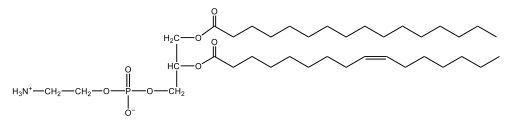
Which one of the following is not a component of phospholipids? So we did talk about phospholipids being involved with fatty acids because it is underneath the umbrella term of fatty acids. Phosphate. We said that phospholipids, a big important aspect of them, is that they contain a phosphate group. It can be connected to a glycerol molecule or a sphingosine molecule. Cholesterol. Cholesterol here is not a component of phospholipids, and in fact, it belongs under the category of steroids. So here, this would be our answer. Then finally, Glycerol. Glycerol can be a vital component of phospholipids. It forms a potential backbone in terms of the attachment of the phosphate group. So this would also be a component of phospholipids. So here the only answer that's correct is option c. Cholesterol is not a component of phospholipids.
Glycerophospholipids Concept 2
Video transcript
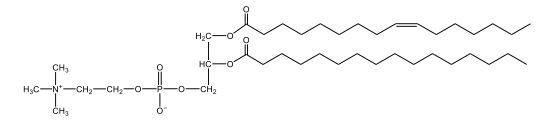
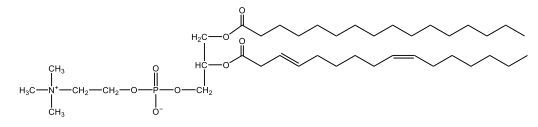
Now, Glycerophospholipids are also called phosphoglycerides, and they are phospholipids with a glycerol backbone and 2 fatty acids. We're going to say here, it has a head and tails, and we're going to say the head portion is a phosphate group extended with an amino alcohol head group. The tail is just 2 fatty acids attached through ester bonds. Now this is important, they're classified based on the head group attached to the phosphate group. If we take a look here, we have two classes that exist. We have Cefalins and then we have Lecithins. Here, when it comes to Cefalins, the head group is an ethanolamine. Here the nitrogen is connected to 3 hydrogens, an ethyl and then followed by an alcohol. This alcohol portion is what bonds to the phosphate group, so, this is the head region. The fatty acid chains could be saturated or unsaturated, again that's not part of the classification. It's the presence of the Ethanolamine group that makes it a Cefalin. Now, Lecithin, here we're going to have choline as our head group. And the clue here, choline CH, instead of having hydrogens that we have methyl groups. And we still have our ethyl and then an OH. That OH is reacting with a phosphate group for the attachment. Now, here we're going to say what's important here and also the fatty acid chains could be saturated or unsaturated. Now, here when it comes to our Glycerophospholipids, they are the most abundant lipids in cell membranes. But remember, the classification of these types of Glycerophospholipids is based on the type of head group.
Glycerophospholipids Example 2
Video transcript
This example question asks, "What is the basis of the classification of Glycerophospholipids?" Now remember, we said that it is the type of head group that's attached to our phosphate that determines the classification for Glycerophospholipids. So we take a look here; it is not about the fatty acid molecule of carbon 1, the number of double bonds in carbon 2 fatty acid, or the fatty acid molecule of carbon 2. Again, it is the head group that's attached to the phosphate group. And remember, here we can have either an ethanolamine head group or a choline head group as the Head Group. In both cases, we have a nitrogen making 4 bonds and therefore it's positively charged. With an ethanolamine head group, we have nitrogen connected to 3 hydrogens, an ethyl group then connected to an alcohol. And then for choline, the nitrogen is not connected to hydrogens but to 3 methyl groups and it still possesses a positive charge. So, in this particular question, it is the head group attached to the phosphate group that deals with the classification of our Glycerophospholipids.
Which one of the following statements accurately describes the difference between cephalins and lecithins?
Cephalins contain saturated fatty acids while lecithins have unsaturated fatty acids.
Lecithins and cephalins have different backbone molecules.
The head groups in cephalins and lecithins are ethanolamine and choline, respectively.
Lecithins do not have a head group.
Glycerophospholipids Example 3
Video transcript
Now, when it comes to drawing Glycerophospholipids, we're going to say it requires recalling the structures of the fatty acids and head groups. Alright. So here it says to draw the structure of a Glycerophospholipid that contains 2 lauric acid acetyl groups and ethanol amine bonded to the phosphate group. Alright. So here, the way we're going to approach this is step 1, we're going to draw the Glycerol backbone with the phosphate group at carbon number 3. So remember, we're going to say this is 123, we're going to say instead of 2 OH groups at carbon 1 and 2, we're going to write only oxygen atoms. So here, carbon 1 and 2 we have an oxygen here and an oxygen here, we already have our phosphate group connected to Carbon 3. Next, we're going to say step 2, extend the Phosphate group at Carbon 3 with, CH 2CH 2 group and an ethyl group. And we're going to say we're going to complete the head group with a NH3+ group because it's ethanolamine, or we're going to complete it with a nitrogen connected to 3 methyl groups, and it's also positive if it's a choline group. So here we're going to say we have CHCH 2 attached. They told us it's an ethanolamine, so it's going to be an NH3+ group attached. And then let's see. So then we're done with that portion. And then finally, it says draw the 2 fatty acyl groups. So here we're going to say, remember, we don't include the OH group of the fatty acid from the 2 oxygen atoms at carbons 1 and 2. Alright. So we have our ethanolamine group attached, and they're telling us within this question it's 2 lauric acid acetyl groups. Remember, lauric acid is going to be a saturated fatty acid. It has 12 carbons in total and it has no pi bonds. So we just have to draw that out. So 2, 4, 6, 8, 10, 12. Same thing here. 2, 4, 6, 8, 10, 12. So here, this would be the structure of our Glycerophospholipid based on the description at the beginning of the example question.
Draw a glycerophospholipid with lauric acid at C1, myristic acid at C2, and choline bonded to phosphate.
Draw a cephalin with stearic acid at C1 and oleic acid at C2.
Draw a lecithin with palmitic acid at C1 and palmitoleic acid at C2.
Do you want more practice?
Here’s what students ask on this topic:
What are glycerophospholipids and why are they important?
Glycerophospholipids, also known as phosphoglycerides, are a type of phospholipid that have a glycerol backbone attached to two fatty acids and a phosphate group. The phosphate group is further extended with an amino alcohol head group. These lipids are amphipathic, meaning they have a hydrophilic (water-attracting) head and a hydrophobic (water-repelling) tail. This unique structure allows them to form lipid bilayers, which are essential components of cell membranes. Glycerophospholipids are crucial for cellular structure and function, as they help maintain the integrity of cell membranes and facilitate various cellular processes, including signaling and transport.
 Created using AI
Created using AIHow are glycerophospholipids classified?
Glycerophospholipids are classified based on the type of head group attached to the phosphate group. The two main classes are cefalin and lecithin. Cefalin has an ethanolamine head group, characterized by a nitrogen atom connected to three hydrogens, an ethyl group, and an alcohol group. Lecithin, on the other hand, has a choline head group, which includes methyl groups instead of hydrogens. The fatty acid chains in both classes can be either saturated or unsaturated, but this does not affect their classification. Understanding these classifications is important for studying their roles in biological systems.
 Created using AI
Created using AIWhat is the role of glycerophospholipids in cell membranes?
Glycerophospholipids play a crucial role in cell membranes by forming the lipid bilayer. Their amphipathic nature, with hydrophilic heads and hydrophobic tails, allows them to arrange themselves in a bilayer with the hydrophilic heads facing the aqueous environment and the hydrophobic tails facing inward. This bilayer structure is essential for maintaining the integrity and functionality of cell membranes. It provides a barrier that separates the cell's interior from its external environment, while also allowing for the selective transport of molecules and facilitating cell signaling processes.
 Created using AI
Created using AIWhat is the difference between cefalin and lecithin?
The primary difference between cefalin and lecithin lies in their head groups. Cefalin has an ethanolamine head group, which includes a nitrogen atom connected to three hydrogens, an ethyl group, and an alcohol group. Lecithin, on the other hand, has a choline head group, characterized by the presence of methyl groups instead of hydrogens. Both types of glycerophospholipids have a glycerol backbone and two fatty acid tails, which can be either saturated or unsaturated. These differences in head groups influence their specific functions and roles within biological membranes.
 Created using AI
Created using AIWhy are glycerophospholipids considered amphipathic?
Glycerophospholipids are considered amphipathic because they contain both hydrophilic (water-attracting) and hydrophobic (water-repelling) regions. The hydrophilic head consists of a phosphate group extended with an amino alcohol, which is polar and interacts well with water. The hydrophobic tail is made up of two fatty acid chains, which are non-polar and do not interact well with water. This dual nature allows glycerophospholipids to form lipid bilayers in aqueous environments, with the hydrophilic heads facing outward towards the water and the hydrophobic tails facing inward, away from the water.
 Created using AI
Created using AIYour GOB Chemistry tutor
- Why are glycerophospholipids more soluble in water than triacylglycerols?
- Draw the structure of the glycerophospholipid that contains a stearic acid acyl group, an oleic acid acyl grou...
- Which of the following terms apply to the compound shown below? (Hint: Look at the functional groups and the b...
- Draw the structure of a glycerophospholipid that contains palmitic acid, oleic acid, and the phosphate bonded ...
- Identify the products formed by complete hydrolysis of all ester bonds in (a) the phosphatidylcholine on page ...
- A membrane lipid was isolated and completely hydrolyzed. The following products were detected: ethanolamine, p...
- Identify the following glycerophospholipid, which is found in the nerves and spinal cord in the body, as a lec...
- Identify the following features of this phospholipid, which is needed for the brain and nerve tissues:<IMAG...
- Palm oil has a high level of glyceryl tripalmitate (tripalmitin). Draw the condensed structural formula for gl...
- Describe the similarities and differences between triacylglycerols and glycerophospholipids.
- Draw the condensed structural formula for the cephalin that contains glycerol, two palmitic acids, phosphate, ...
- Draw the condensed structural formula for a glycerophospholipid that contains glycerol, two stearic acids, pho...
- Mayonnaise is a thick mixture containing oil, vinegar (water-based), and eggs; the eggs contain a phospholipid...
- Compare the structure of a soap molecule to a phospholipid and explain why a soap’s polar head is smaller than...