Enzymes are biological catalysts that facilitate various biochemical reactions, and they are classified into six main classes based on the type of reaction they catalyze. Understanding these classes is essential for grasping how enzymes function in metabolic processes.
The first class, oxidoreductases, is involved in redox reactions, where electrons are transferred between molecules. For instance, in a reaction where molecule A loses two electrons to molecule B, A is oxidized while B is reduced. This transfer of electrons is fundamental in energy production and metabolic pathways.
Next, we have transferases, which catalyze the transfer of functional groups from one molecule to another. In this process, a specific group from molecule A is transferred to molecule B, resulting in a modified structure of B. This class plays a crucial role in various biosynthetic pathways.
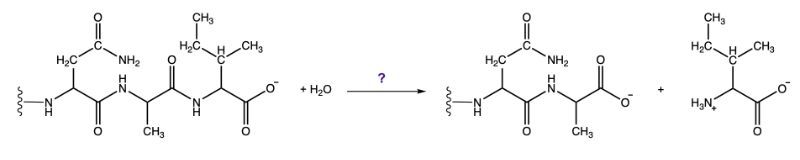
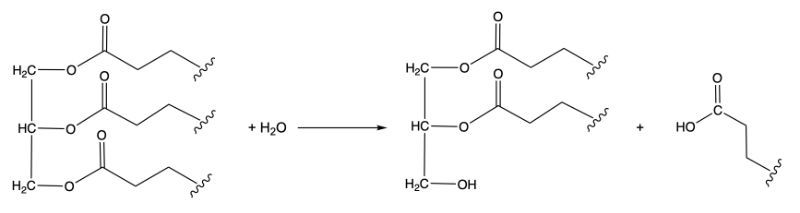
The third class, hydrolases, involves the cleavage of bonds through the addition of water. For example, when water is added to a bond between molecules A and B, it facilitates the breaking of that bond, resulting in the formation of new products. This reaction is vital in digestion and metabolism.
Isomerases are the fourth class, responsible for rearranging atoms within a molecule to create isomers. Isomers have the same molecular formula but differ in the arrangement of their atoms, which can significantly affect their properties and functions in biological systems.
The fifth class, lyases, catalyzes the breaking or forming of bonds without involving redox reactions or the addition of water. This class is important for various metabolic pathways, as it allows for the synthesis and breakdown of complex molecules.
Finally, ligases require energy, often in the form of ATP, to join two molecules together. This process is essential for DNA replication and repair, as well as for the synthesis of larger biomolecules.
In summary, the six classes of enzymes—oxidoreductases, transferases, hydrolases, isomerases, lyases, and ligases—each play distinct and vital roles in biochemical reactions, facilitating the complex processes necessary for life.