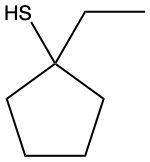
Here we're going to say that thiols, also called mercaptans, possess a mercapto group. "Mercapto" just means SH group, connected to a carbon atom. Now the set of rules for naming thiols is very similar to the ones used to name alcohols. Here, all we do is add "thiol" to the parent chain name. So our naming convention will be the location of our different substituents, then we have the location and then the parent, and then our final name. Right? This is the way we would approach naming different types of files.
- 1. Matter and Measurements4h 29m
- What is Chemistry?5m
- The Scientific Method9m
- Classification of Matter16m
- States of Matter8m
- Physical & Chemical Changes19m
- Chemical Properties8m
- Physical Properties5m
- Intensive vs. Extensive Properties13m
- Temperature (Simplified)9m
- Scientific Notation13m
- SI Units (Simplified)5m
- Metric Prefixes24m
- Significant Figures (Simplified)11m
- Significant Figures: Precision in Measurements7m
- Significant Figures: In Calculations19m
- Conversion Factors (Simplified)15m
- Dimensional Analysis22m
- Density12m
- Specific Gravity9m
- Density of Geometric Objects19m
- Density of Non-Geometric Objects9m
- 2. Atoms and the Periodic Table5h 23m
- The Atom (Simplified)9m
- Subatomic Particles (Simplified)12m
- Isotopes17m
- Ions (Simplified)22m
- Atomic Mass (Simplified)17m
- Atomic Mass (Conceptual)12m
- Periodic Table: Element Symbols6m
- Periodic Table: Classifications11m
- Periodic Table: Group Names8m
- Periodic Table: Representative Elements & Transition Metals7m
- Periodic Table: Elemental Forms (Simplified)6m
- Periodic Table: Phases (Simplified)8m
- Law of Definite Proportions9m
- Atomic Theory9m
- Rutherford Gold Foil Experiment9m
- Wavelength and Frequency (Simplified)5m
- Electromagnetic Spectrum (Simplified)11m
- Bohr Model (Simplified)9m
- Emission Spectrum (Simplified)3m
- Electronic Structure4m
- Electronic Structure: Shells5m
- Electronic Structure: Subshells4m
- Electronic Structure: Orbitals11m
- Electronic Structure: Electron Spin3m
- Electronic Structure: Number of Electrons4m
- The Electron Configuration (Simplified)22m
- Electron Arrangements5m
- The Electron Configuration: Condensed4m
- The Electron Configuration: Exceptions (Simplified)12m
- Ions and the Octet Rule9m
- Ions and the Octet Rule (Simplified)8m
- Valence Electrons of Elements (Simplified)5m
- Lewis Dot Symbols (Simplified)7m
- Periodic Trend: Metallic Character4m
- Periodic Trend: Atomic Radius (Simplified)7m
- 3. Ionic Compounds2h 18m
- Periodic Table: Main Group Element Charges12m
- Periodic Table: Transition Metal Charges6m
- Periodic Trend: Ionic Radius (Simplified)5m
- Periodic Trend: Ranking Ionic Radii8m
- Periodic Trend: Ionization Energy (Simplified)9m
- Periodic Trend: Electron Affinity (Simplified)8m
- Ionic Bonding6m
- Naming Monoatomic Cations6m
- Naming Monoatomic Anions5m
- Polyatomic Ions25m
- Naming Ionic Compounds11m
- Writing Formula Units of Ionic Compounds7m
- Naming Ionic Hydrates6m
- Naming Acids18m
- 4. Molecular Compounds2h 18m
- Covalent Bonds6m
- Naming Binary Molecular Compounds6m
- Molecular Models4m
- Bonding Preferences6m
- Lewis Dot Structures: Neutral Compounds (Simplified)8m
- Multiple Bonds4m
- Multiple Bonds (Simplified)6m
- Lewis Dot Structures: Multiple Bonds10m
- Lewis Dot Structures: Ions (Simplified)8m
- Lewis Dot Structures: Exceptions (Simplified)12m
- Resonance Structures (Simplified)5m
- Valence Shell Electron Pair Repulsion Theory (Simplified)4m
- Electron Geometry (Simplified)8m
- Molecular Geometry (Simplified)11m
- Bond Angles (Simplified)11m
- Dipole Moment (Simplified)15m
- Molecular Polarity (Simplified)7m
- 5. Classification & Balancing of Chemical Reactions3h 17m
- Chemical Reaction: Chemical Change5m
- Law of Conservation of Mass5m
- Balancing Chemical Equations (Simplified)13m
- Solubility Rules16m
- Molecular Equations18m
- Types of Chemical Reactions12m
- Complete Ionic Equations18m
- Calculate Oxidation Numbers15m
- Redox Reactions17m
- Spontaneous Redox Reactions8m
- Balancing Redox Reactions: Acidic Solutions17m
- Balancing Redox Reactions: Basic Solutions17m
- Balancing Redox Reactions (Simplified)13m
- Galvanic Cell (Simplified)16m
- 6. Chemical Reactions & Quantities2h 35m
- 7. Energy, Rate and Equilibrium3h 46m
- Nature of Energy6m
- First Law of Thermodynamics7m
- Endothermic & Exothermic Reactions7m
- Bond Energy14m
- Thermochemical Equations12m
- Heat Capacity19m
- Thermal Equilibrium (Simplified)8m
- Hess's Law23m
- Rate of Reaction11m
- Energy Diagrams12m
- Chemical Equilibrium7m
- The Equilibrium Constant14m
- Le Chatelier's Principle23m
- Solubility Product Constant (Ksp)17m
- Spontaneous Reaction10m
- Entropy (Simplified)9m
- Gibbs Free Energy (Simplified)18m
- 8. Gases, Liquids and Solids3h 25m
- Pressure Units6m
- Kinetic Molecular Theory14m
- The Ideal Gas Law18m
- The Ideal Gas Law Derivations13m
- The Ideal Gas Law Applications6m
- Chemistry Gas Laws16m
- Chemistry Gas Laws: Combined Gas Law12m
- Standard Temperature and Pressure14m
- Dalton's Law: Partial Pressure (Simplified)13m
- Gas Stoichiometry18m
- Intermolecular Forces (Simplified)19m
- Intermolecular Forces and Physical Properties11m
- Atomic, Ionic and Molecular Solids10m
- Heating and Cooling Curves30m
- 9. Solutions4h 10m
- Solutions6m
- Solubility and Intermolecular Forces18m
- Solutions: Mass Percent6m
- Percent Concentrations10m
- Molarity18m
- Osmolarity15m
- Parts per Million (ppm)13m
- Solubility: Temperature Effect8m
- Intro to Henry's Law4m
- Henry's Law Calculations12m
- Dilutions12m
- Solution Stoichiometry14m
- Electrolytes (Simplified)13m
- Equivalents11m
- Molality15m
- The Colligative Properties15m
- Boiling Point Elevation16m
- Freezing Point Depression9m
- Osmosis16m
- Osmotic Pressure9m
- 10. Acids and Bases3h 29m
- Acid-Base Introduction11m
- Arrhenius Acid and Base6m
- Bronsted Lowry Acid and Base18m
- Acid and Base Strength17m
- Ka and Kb12m
- The pH Scale19m
- Auto-Ionization9m
- pH of Strong Acids and Bases9m
- Acid-Base Equivalents14m
- Acid-Base Reactions7m
- Gas Evolution Equations (Simplified)6m
- Ionic Salts (Simplified)23m
- Buffers25m
- Henderson-Hasselbalch Equation16m
- Strong Acid Strong Base Titrations (Simplified)10m
- 11. Nuclear Chemistry56m
- BONUS: Lab Techniques and Procedures1h 38m
- BONUS: Mathematical Operations and Functions47m
- 12. Introduction to Organic Chemistry1h 34m
- 13. Alkenes, Alkynes, and Aromatic Compounds2h 12m
- 14. Compounds with Oxygen or Sulfur1h 6m
- 15. Aldehydes and Ketones1h 1m
- 16. Carboxylic Acids and Their Derivatives1h 11m
- 17. Amines38m
- 18. Amino Acids and Proteins1h 51m
- 19. Enzymes1h 37m
- 20. Carbohydrates1h 46m
- Intro to Carbohydrates4m
- Classification of Carbohydrates4m
- Fischer Projections4m
- Enantiomers vs Diastereomers8m
- D vs L Enantiomers8m
- Cyclic Hemiacetals8m
- Intro to Haworth Projections4m
- Cyclic Structures of Monosaccharides11m
- Mutarotation4m
- Reduction of Monosaccharides10m
- Oxidation of Monosaccharides7m
- Glycosidic Linkage14m
- Disaccharides7m
- Polysaccharides7m
- 21. The Generation of Biochemical Energy2h 8m
- 22. Carbohydrate Metabolism2h 22m
- 23. Lipids2h 26m
- Intro to Lipids6m
- Fatty Acids25m
- Physical Properties of Fatty Acids6m
- Waxes4m
- Triacylglycerols12m
- Triacylglycerol Reactions: Hydrogenation8m
- Triacylglycerol Reactions: Hydrolysis13m
- Triacylglycerol Reactions: Oxidation7m
- Glycerophospholipids15m
- Sphingomyelins13m
- Steroids15m
- Cell Membranes7m
- Membrane Transport10m
- 24. Lipid Metabolism1h 45m
- 25. Protein and Amino Acid Metabolism1h 37m
- 26. Nucleic Acids and Protein Synthesis2h 54m
- Intro to Nucleic Acids4m
- Nitrogenous Bases16m
- Nucleoside and Nucleotide Formation9m
- Naming Nucleosides and Nucleotides13m
- Phosphodiester Bond Formation7m
- Primary Structure of Nucleic Acids11m
- Base Pairing10m
- DNA Double Helix6m
- Intro to DNA Replication20m
- Steps of DNA Replication11m
- Types of RNA10m
- Overview of Protein Synthesis4m
- Transcription: mRNA Synthesis9m
- Processing of pre-mRNA5m
- The Genetic Code6m
- Introduction to Translation7m
- Translation: Protein Synthesis18m
Naming Thiols: Study with Video Lessons, Practice Problems & Examples
 Created using AI
Created using AIThiols, or mercaptans, contain a mercapto group (–SH) attached to a carbon atom. Their naming conventions resemble those of alcohols, where "thiol" is added to the parent chain name. The structure includes the location of substituents followed by the parent name. Understanding thiols is essential in organic chemistry, particularly in reactions involving functional groups like aldehydes and ketones, and their role in biochemical processes such as alcoholic fermentation and enzyme activation.
Rules for Naming Thiols Concept 1
Video transcript
Naming Thiols Example 1
Video transcript
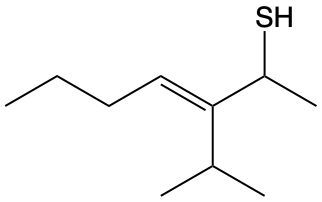
Name the following thiol compound. Right. So step 1 says to find the longest carbon chain because it'll represent the parent chain, and assign a name according to the prefixes. Now, the parent chain should include the SH group, the mercapto group, and have the largest number of carbons. If there is a tie between the longest chain, choose the chain with more substituents. If we take a look here, our longest chain would have to be this chain. Now we have our longest chain, so we have to, let's see, assign names according to the prefix. We're going to assign names to all the substituents here. So we know that this SH group is going to become a thiol. It's going to form near the end of the name, so we won't worry about it yet. We have "br" here, which is bromo, and one carbon here is methyl.
Step 3 is we start numbering the chain from the end closest to the SH group. So the end close to the SH group is here: 1, 2, 3, 4, 5, 6. If there's a tie, number from the end closest to the next substituent. If it's still a tie, number in alphabetical order. Assign the numerical location to the carbon with the SH group, which we did. And then steps 4 to 6 will repeat steps from previous naming topics. So here this is what it would look like. Alright, so we'd say we have bromo and methyl. We name those alphabetically. So bromo is on 3, so 3-bromo. 5-methyl. Then we're going to say that the thiol group, the mercapto group, is on carbon 2, so 2. It's a 6-carbon chain, so hexanethiol. Okay? So this would be the name of this particular thiol compound.
Determine IUPAC name for given compound.
1,2-hexenedithiol
5,6-hexanedithiol
5,6-heptanedithiol
1,2-hexanedithiol
Determine IUPAC name for given compound.
3-heptene-3-isopropyl-2-thiol
3-isobutyl-4-heptene-2-thiol
3-isopropyl-3-heptene-2-thiol
4-heptene-3-isobutyl-2-thiol
Draw skeletal structural formula for 2-ethylcyclopentanethiol.
Do you want more practice?
Here’s what students ask on this topic:
What are thiols and how are they named?
Thiols, also known as mercaptans, are organic compounds that contain a mercapto group (–SH) attached to a carbon atom. The naming conventions for thiols are similar to those for alcohols. To name a thiol, you add the suffix 'thiol' to the parent chain name. The structure includes the location of substituents followed by the parent name and then 'thiol'. For example, if the SH group is attached to the second carbon of a propane chain, it would be named 2-propanethiol.
 Created using AI
Created using AIHow do you differentiate between thiols and alcohols in naming?
While both thiols and alcohols have similar naming conventions, the key difference lies in the functional group. Alcohols contain a hydroxyl group (–OH), whereas thiols contain a mercapto group (–SH). In naming, alcohols use the suffix 'ol' (e.g., ethanol), while thiols use the suffix 'thiol' (e.g., ethanethiol). The position of the functional group is indicated by a number, and the rest of the naming follows the standard IUPAC rules for organic compounds.
 Created using AI
Created using AIWhat is the significance of thiols in biochemical processes?
Thiols play a crucial role in various biochemical processes. They are involved in the formation of disulfide bonds, which are important for the structural stability of proteins. Thiols also participate in redox reactions, acting as antioxidants. In metabolic pathways, thiols are essential in enzyme activation and in processes like alcoholic fermentation. Their ability to form strong bonds with heavy metals makes them important in detoxification processes as well.
 Created using AI
Created using AICan you provide an example of naming a thiol with multiple substituents?
Sure! Let's consider a thiol with the molecular structure CH3-CH(SH)-CH2-CH3. Here, the SH group is attached to the second carbon of a butane chain. Additionally, if there is a methyl group attached to the third carbon, the compound would be named 3-methyl-2-butanethiol. The numbering ensures the lowest possible locants for the substituents and the thiol group.
 Created using AI
Created using AIWhat are some common uses of thiols in industry?
Thiols are widely used in various industries due to their unique properties. In the petroleum industry, they are used as odorants for natural gas to detect leaks. Thiols are also used in the production of certain pharmaceuticals and agrochemicals. In the rubber industry, they act as vulcanization agents. Additionally, thiols are used in the synthesis of polymers and as intermediates in organic synthesis.
 Created using AI
Created using AI