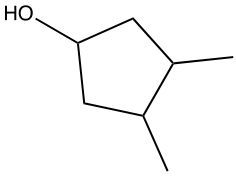
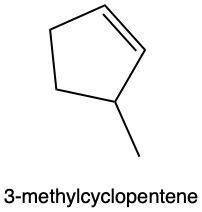
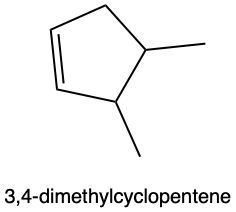
In hydration reactions, we have the acid-catalyzed addition of water to an alkene, and it produces an alcohol. In this reaction, we're going to add 1 H and 1 OH, both coming from water, to 1 pi bond. If we take a look at the overview for this reaction, we start with an alkene, and we're going to add to it a water molecule. To do that, we need to utilize sulfuric acid, which acts as an acid catalyst to get the process going. Now, if we look at the alkene, both of these double-bonded carbons are the same. The alkene is symmetrical. They both are connected to 1 carbon and 1 H. That means that the H and OH can go to either of those double-bonded carbons. H goes to one, OH goes to the other one.
Later on, we'll see what happens when we have an alkene that is not symmetrical. Where would the H go in that case, where would the OH go? So, if we take a look, I'm going to add the H onto this double-bonded carbon. We're going to break the double bond in order to add it, so at the end we're not going to have a double bond within our product. And then we have our OH added to the other one. This produces our alcohol. So just remember, a hydration reaction means we're adding water. Here we're adding a water molecule to an alkene in order to produce an alcohol.