8. Gases, Liquids and Solids
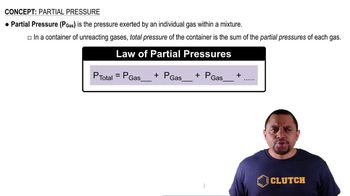
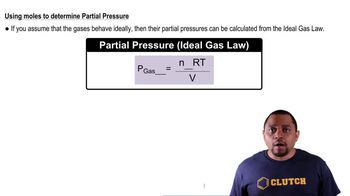
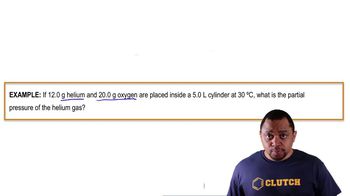
Dalton's Law: Partial Pressure (Simplified)
8. Gases, Liquids and Solids
Dalton's Law: Partial Pressure (Simplified)
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
A gas mixture contains 72.8% chlorine and 27.2% neon by mass. What is the partial pressure of neon in the mixture if the total pressure is recorded as 809 mmHg?
274views2rank1comments - Multiple Choice
The partial pressure of N2 in the air is 593 mmHg at 1 atm. What is the partial pressure of N2 in a bubble of air a scuba diver breathes when he is 66 ft below the surface of the water where the pressure is 3.00 atm?
691views1rank - Textbook QuestionDetermine the percent composition of air in the lungs from the following composition in partial pressures: PN2 = 573 mmHg, PO2 = 100 mmHg, PCO2 = 40 mmHg, and PH2O = 47 mmHg; all at 37 °C and 1 atm pressure.525views
- Textbook QuestionSuppose a mixture contains helium and oxygen gases. If the partial pressure of helium is the same as the partial pressure of oxygen, what do you know about the number of helium atoms compared to the number of oxygen molecules? Explain.263views
- Textbook QuestionIn certain lung ailments such as emphysema, there is a decrease in the ability of oxygen to diffuse into the blood. b. Why does a person with severe emphysema sometimes use a portable oxygen tank?183views
- Textbook QuestionIn certain lung ailments such as emphysema, there is a decrease in the ability of oxygen to diffuse into the blood. a. How would the partial pressure of oxygen in the blood change?215views