5. Classification & Balancing of Chemical Reactions
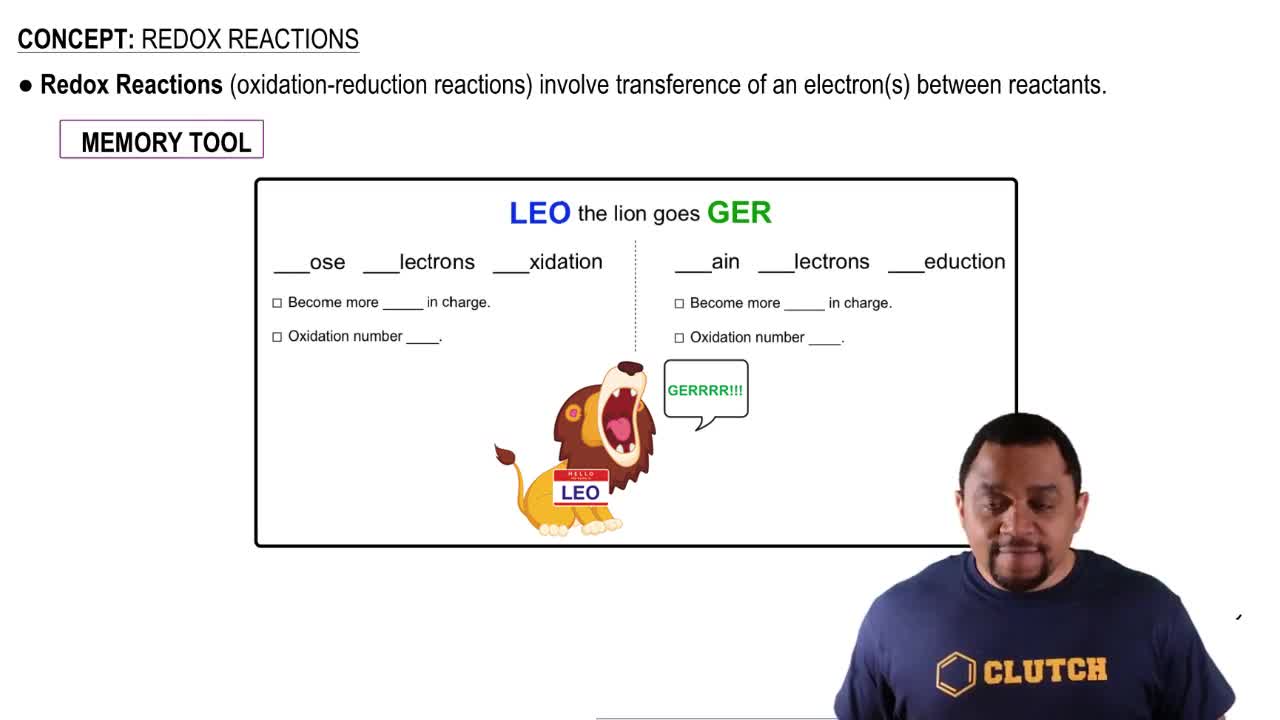
Redox Reactions
5. Classification & Balancing of Chemical Reactions
Redox Reactions
Practice this topic
- Multiple Choice
Which element is being reduced in the following reaction?
Cr2O72- + 3 HNO2 + 5 H+ → 2 Cr3+ + 3NO3- + 4 H2O
1946views2rank - Multiple Choice
Identify the oxidizing agent and reducing agent from the following redox reaction.
Ba (s) + Cl2 (g) → BaCl2 (aq)
2475views7rank - Multiple Choice
Which element is oxidized and which is reduced in the following reaction?
Hg (aq) + HgCl2 (aq) → Hg2Cl2
1373views5rank - Multiple Choice
Which of the following represents an oxidation-reduction reaction?
I. PCl3 (aq) + Cl2 (g) → PCl5 (aq)
II. 2 AgNO3 (aq) + Cu (s) → Cu(NO3)2 (aq) + 2 Ag (s)
III. CO2 (g) + 2 LiOH (aq) → Li2CO3 (aq) + H2O (l)
IV. FeCl2 (aq) + 2 NaOH (aq) → Fe(OH)2 (aq) + 2 NaCl (aq)
1500views11rank - Textbook QuestionIdentify the oxidized reactant, the reduced reactant, the oxidizing agent, and the reducing agent in the following reactions: a. Fe(s)+Cu₂+(aq) → Fe₂+(aq)+Cu(s) b. Mg(s)+Cl₂(g) → MgCl₂(s) c. 2 Al(s)+Cr₂O₃(s) → 2 Cr(s) + Al₂O₃(s)550views
- Textbook QuestionPotassium, a silvery metal, reacts with bromine, a corrosive, reddish liquid, to yield potassium bromide, a white solid. Write the balanced equation, and identify the oxidizing and reducing agents.770views
- Textbook QuestionIdentify each of the following as an oxidation or a reduction: c. Cr³⁺(aq) + 3e⁻ → Cr(s)274views
- Textbook QuestionIn the mitochondria of human cells, energy is provided by the oxidation and reduction reactions of the iron ions in the cytochromes in electron transport. Identify each of the following as an oxidation or a reduction: a. Fe³⁺ + e⁻ → Fe²⁺306views