15. Aldehydes and Ketones
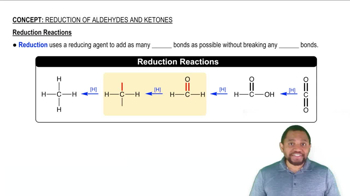
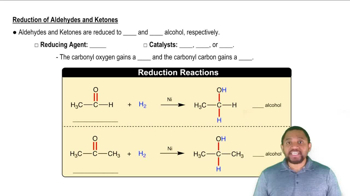
Reduction of Aldehydes and Ketones
Practice this topic
- Multiple Choice
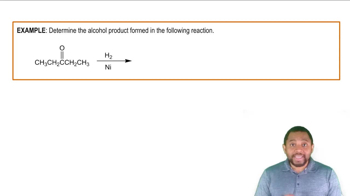
Determine the alcohol product formed in the following reaction.
119views - Multiple Choice
Determine which reactant should be used to produce the following alcohol.
155views - Textbook Question
The carbonyl group can be reduced by addition of a hydride ion (H⁻) and (H⁺) a proton
. Removal of H⁻ and H⁺ from an alcohol results in a carbonyl group <IMAGE>
a.To which atom of the carbonyl is the hydride ion added and why?
144views - Textbook Question
The carbonyl group can be reduced by addition of a hydride ion (H⁻) and (H⁺) a proton
. Removal of H⁻ and H⁺ from an alcohol results in a carbonyl group <IMAGE>
b. In the reaction, indicate which direction represents reduction and which represents oxidation.
177views - Textbook Question
Write the structures of the hemiacetal or hemiketal that result from reactions (a) and (b). Write the structures of the complete hydrolysis products of the acetal or ketal in (c) and (d).
Acetone + Ethanol → ?
152views - Textbook Question
What ketones or aldehydes might be reduced to yield the following alcohols?
a. <IMAGE>
b. <IMAGE>
c. HOCH2―CH2―CH2OH
149views