Here are the essential concepts you must grasp in order to answer the question correctly.
Acids
Acids are substances that can donate protons (H+) in a chemical reaction. They typically have a sour taste and can turn blue litmus paper red. Acids react with bases to form water and salts in a neutralization reaction, which is a key characteristic of their behavior.
Recommended video:
Bases
Bases are substances that can accept protons or donate hydroxide ions (OH-) in a chemical reaction. They usually have a bitter taste and can turn red litmus paper blue. Bases neutralize acids, resulting in the formation of water and salts, which is a fundamental property of bases.
Recommended video:
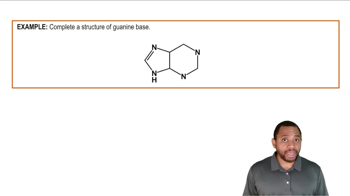
Nitrogenous Bases Example 3
Neutralization Reaction
A neutralization reaction occurs when an acid reacts with a base to produce water and a salt. This process typically results in the cancellation of the acidic and basic properties, leading to a solution that is closer to neutral pH. The statement 'neutralizes bases' indicates that the substance in question is likely an acid, as acids are known for this characteristic.
Recommended video:
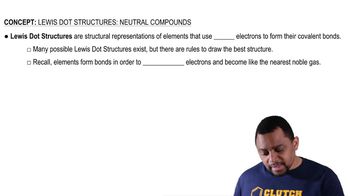
Lewis Dot Structures: Neutral Compounds (Simplified) Concept 1
 Verified Solution
Verified Solution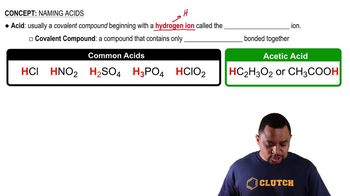

 0:40m
0:40m
