Here are the essential concepts you must grasp in order to answer the question correctly.
Polyamide Formation
Polyamides are a type of polymer formed through the reaction of diamines and dicarboxylic acids. In this case, ethylenediamine, a diamine, reacts with oxalic acid, a dicarboxylic acid, to create a repeating unit that links together to form a long-chain polymer. Understanding this reaction is crucial for visualizing the structure of the resulting polyamide.
Recommended video:
Amide Formation Concept 1
Condensation Reaction
The formation of polyamides involves a condensation reaction, where two molecules combine to form a larger molecule while releasing a small molecule, typically water. This process is essential in polymer chemistry as it leads to the formation of covalent bonds between monomers, resulting in a stable polymer structure. Recognizing this reaction type helps in predicting the byproducts and the overall reaction mechanism.
Recommended video:
Condensed Formula Concept 1
Structural Representation of Polymers
Drawing the polymer formed from the reaction requires an understanding of how to represent the repeating units and the overall structure of the polymer. This involves illustrating the backbone of the polymer, which consists of the alternating units derived from ethylenediamine and oxalic acid, and ensuring that the functional groups are correctly depicted to reflect the chemical nature of the polymer.
Recommended video:
Molecular Representations Concept 1
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: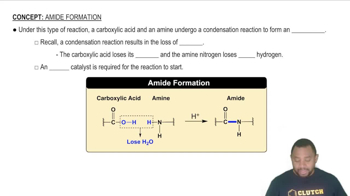



 1:9m
1:9m