Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Shells
Electron shells are the regions around an atom's nucleus where electrons are likely to be found. Each shell is associated with a principal quantum number (n), which indicates its energy level and distance from the nucleus. The third shell corresponds to n=3, while the fourth shell corresponds to n=4.
Recommended video:
Electronic Structure: Shells Concept 1
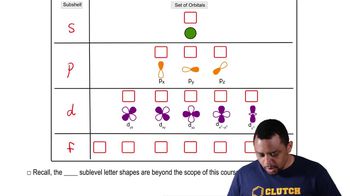
Orbitals
Orbitals are specific regions within electron shells where electrons are likely to be located. Each orbital can hold a maximum of two electrons with opposite spins. The types of orbitals (s, p, d, f) determine their shapes and the number of orbitals in each shell increases with the principal quantum number.
Recommended video:
Electronic Structure: Orbitals Concept 3
Calculating Orbitals in Shells
The total number of orbitals in a shell can be calculated using the formula n^2, where n is the principal quantum number of the shell. For the third shell (n=3), there are 3^2 = 9 orbitals, and for the fourth shell (n=4), there are 4^2 = 16 orbitals. This calculation helps in understanding the electron configuration of elements.
Recommended video:
Electronic Structure: Orbitals Concept 2
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 0:46m
0:46m