Here are the essential concepts you must grasp in order to answer the question correctly.
Solute and Solvent
In a solution, the solute is the substance that is dissolved, while the solvent is the medium in which the solute dissolves. For example, in a saltwater solution, salt is the solute and water is the solvent. Understanding the roles of solutes and solvents is crucial for analyzing how solutions form and behave.
Recommended video:
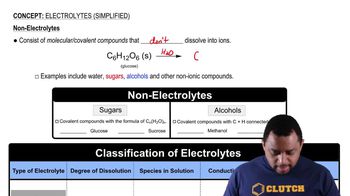
Nonelectrolyte
A nonelectrolyte is a substance that does not dissociate into ions when dissolved in a solvent, meaning it does not conduct electricity. Common examples include sugar and alcohol. Recognizing whether a solute is a nonelectrolyte helps in predicting the properties of the solution, such as its conductivity and boiling point.
Recommended video:
Electrolytes (Simplified) Concept 3
Molecular Representation
Molecular representation involves using diagrams or models to illustrate the structure and interactions of molecules in a solution. The blue and red spheres in the provided image likely represent different types of molecules, helping visualize how solutes interact with solvents. This concept is essential for understanding the physical and chemical properties of solutions.
Recommended video:
Molecular Representations Concept 1

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:38m
2:38m