Here are the essential concepts you must grasp in order to answer the question correctly.
Mass Percent (m/m)
Mass percent (m/m) is a way to express the concentration of a component in a solution, calculated as the mass of the solute divided by the total mass of the solution, multiplied by 100. In this context, it helps determine how much of the NaCl solution is made up of NaCl by comparing the mass of NaCl to the total mass of the solution.
Recommended video:
Evaporation and Mass Loss
Evaporation is the process where liquid turns into vapor, often resulting in a loss of mass when a solute is dissolved in a solvent. In this experiment, heating the NaCl solution causes the water to evaporate, leaving behind solid NaCl, which is crucial for calculating the mass percent of the solution.
Recommended video:
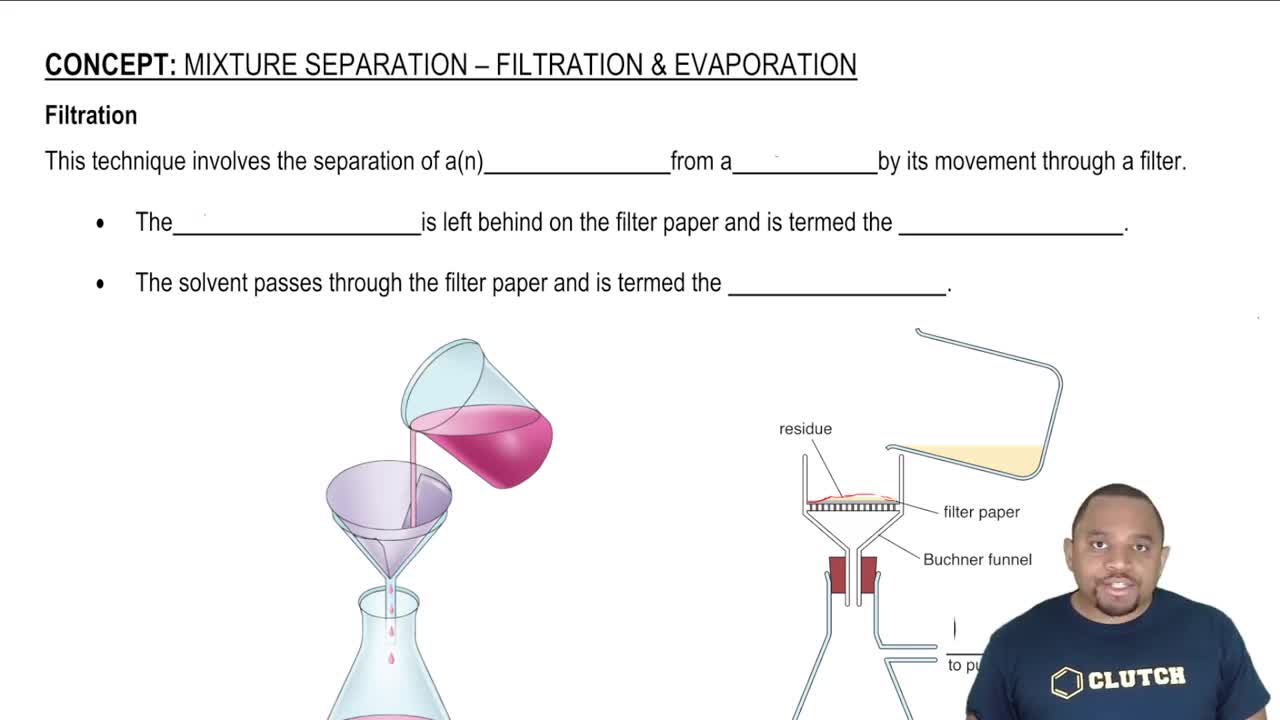
Filtration and Evaporation
Calculating Mass of Solute
To find the mass of the solute (NaCl) in the solution, one must subtract the mass of the evaporating dish and dry NaCl from the mass of the dish with the NaCl solution. This calculation is essential for determining the mass percent, as it provides the necessary mass of NaCl to use in the mass percent formula.
Recommended video:
Solutions: Mass Percent Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:29m
1:29m